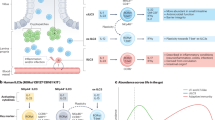
Abstract
The intestine plays a fundamental role as a regulator of the mucosal immune response, mostly through the production and secretion of secretory Immunoglobulin A (sIgA) by the gut-associated lymphoid tissue (GALT). Enteral stimulation, a balance between the commensal microbiota and pathogenic microorganisms, in addition to an adequate nutritional status is required for the optimal immune function of the intestine. Fasting subjects or those supported only with parenteral nutrition, show a progressive anatomical and physiological deterioration of the GALT, triggering a series of alterations resulting in a decrease in the intestinal immune response, modification in the type of microbiota, and changes that lead to or aggravate malnutrition. Patients with malnutrition present an increase in the rate of nosocomial infections, hospital length of stay, and mortality. An adequate nutritional assessment at hospital admission and avoiding long periods of fasting are paramount to prevent these unfavorable outcomes. Herein, we present a mini-state of the art review on the role and importance of enteral stimulation by GALT-mediated immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? J Parenter Enter Nutr. 2007;31:246–58.
Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34:599–608.
Elmore SA. Enhanced histopathology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34:687–96.
Fukatsu K, Kudsk KA. Nutrition and gut immunity. Surg Clin North Am. 2011;91:755–70.
Pierre JF. Gastrointestinal immune and microbiome changes during parenteral. Am J Physiol - Gastrointest Liver Physiol. 2017;312:G246–56.
Pierre JF, Busch RA, Kudsk KA. The gastrointestinal immune system: implications for the surgical patient. Curr Probl Surg. 2016;53:11–47.
Jonker MA, Hermsen JL, Sano Y, Heneghan AF, Lan J, Kudsk KA. Small intestine mucosal immune system response to injury and the impact of parenteral nutrition. Surg [Internet] 2012;151:278–86.
Szefel J, Kruszewski WJ, Buczek T. Enteral feeding and its impact on the gut immune system and intestinal mucosal barrier. Prz Gastroenterol. 2015;10:71–7.
Gommerman JL, Rojas OL, Fritz JH. Re-thinking the functions of IgA+plasma cells. Gut Microbes. 2015;5:652–62.
Woof JM, Ken MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–82.
Anastasilakis CD, Ioannidis O, Gkiomisi AI, Botsios D. Artificial nutrition and intestinal mucosal barrier functionality. Digestion. 2013;88:193–208.
Suzuki K, Fagarasan S. How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008;29:523–31.
Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–32.
Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, et al. Enteral versus parenteral feeding effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–13.
Sano Y, Gomez FE, Kang W, Lan J, Maeshima Y, Hermsen JL, et al. Intestinal polymeric immunoglobulin receptor is affected by type and route of nutrition. J Parenter Enter Nutr. 2007;31:356–7.
Hermsen JL, Sano Y, Gomez FE, Maeshima Y, Kang W, Kudsk KA. Parenteral nutrition inhibits tumor necrosis factor- α-mediated IgA response to injury. Surg Infect (Larchmt) 2008;9:33–40.
Heneghan AF, Pierre JF, Tandee K, Shanmuganayagam D, Wang X, Reed JD, et al. Parenteral nutrition decreases paneth cell function and intestinal bactericidal activity while increasing susceptibility to bacterial enteroinvasion. J Parenter Enter Nutr. 2014;38:817–24.
Ikeda S, Kudsk KA, Fukatsu K, Johnson CD, Le T, Reese S, et al. Enteral feeding preserves mucosal immunity despite in vivo MAdCAM-1 blockade of lymphocyte homing. Ann Surg. 2003;237:677–85.
Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma - Inj Infect Crit Care. 1995;39:44–51.
Hermsen JL, Sano Y, Kudsk KA. Food fight! Parenteral nutrition, enteral stimulation and gut-derived mucosal immunity. Langenbeck’s Arch Surg. 2009;394:17–30.
Fukatsu K. Role of nutrition in gastroenterological surgery. Ann Gastroenterol Surg. 2019;3:160–8.
Fukatsu K. Impact of the feeding route on gut mucosal immunity. Curr Opin Clin Nutr Metab Care. 2014;17:164–70.
Elke G, van Zanten ARH, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care/ 2016;20:1–14.
McCoy KD, Burkhard R, Geuking MB. The microbiome and immune memory formation. Immunol Cell Biol. 2019;97:625–35.
Huus KE, Bauer KC, Brown EM, Bozorgmehr T, Woodward SE, Serapio-Palacios A, et al. Commensal bacteria modulate immunoglobulin a binding in response to host nutrition. Cell Host Microbe. 2020;27:1–13.
Miyasaka EA, Feng Y, Poroyko V, Falkowski NR, Erb-Downward J, Gillilland MG, et al. Total parenteral nutrition–associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. J Immunol. 2013;190:6607–15.
Amarasinghe JJ, D’Hondt RE, Waters CM, Mantis NJ. Exposure of Salmonella enterica serovar typhimurium to a protective monoclonal IGA triggers exopolysaccharide production via a diguanylate cyclase-dependent pathway. Infect Immun. 2013;81:653–64.
Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, Werner JJ, et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14:571–81.
McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). J Parenter Enter Nutr. 2016;40:159–211.
Okamoto K, Fukatsu K, Hashiguchi Y, Ueno H, Shinto E, Moriya T, et al. Lack of preoperative enteral nutrition reduces gut-associated lymphoid cell numbers in colon cancer patients: a possible mechanism underlying increased postoperative infectious complications during parenteral nutrition. Ann Surg. 2013;258:1059–64.
Patel JJ, Kozeniecki M, Biesboer A, Peppard W, Ray AS, Thomas S, et al. Early trophic enteral nutrition is associated with improved outcomes in mechanically ventilated patients with septic shock: a retrospective review. J Intensive Care Med. 2016;31:471–7.
Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2013;37:259–84.
Higashizono K, Fukatsu K, Watkins A, Watanabe T, Noguchi M, Ri M, et al. Influences of short-term fasting and carbohydrate supplementation on gut immunity and mucosal morphology in mice. J Parenter Enter Nutr. 2019;43:516–24.
Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37:386–98.
Correia MITD, Perman MI, Waitzberg DL. Hospital malnutrition in Latin America: a systematic review. Clin Nutr. 2017;36:958–67.
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T. et al. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9.
Pérez Romero MT, Serralde-Zuniga AE, Reyes Ramírez AL, del C, Alfonso Baruch E, Gulías Herrero A. et al. Prevalence of malnutrition at admission in hospitalized adults at INCMNSZ in Mexico City. Rev Mex Endocrinol Metab y Nutr. 2017;4:12–6.
Waitzberg DL, Ravacci GR, Raslan M. Desnutrición hospitalaria. Nutr Hosp. 2011;26:254–64.
Rangel AG, Sepúlveda FR, Domínguez RS, Maldonado GM. Evaluación del estado nutricional y su impacto en pacientes post operados de anastomosis intestinal. Nutrición y fuga anastomosis. Nutr Clin y Diet Hosp. 2016;36:82–8.
Morris HJ, Carrillo OV, Llauradó G, Alonso ME, Bermúdez RC, Lebeque Y, et al. Effect of starvation and refeeding on biochemical and immunological status of Balb/c mice: an experimental model of malnutrition. Immunopharmacol Immunotoxicol. 2011;33:438–46.
Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med. 2015;7:276ra24.
Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schütz T, et al. ESPEN guidelines on enteral nutrition: gastroenterology. Clin Nutr. 2006;25:260–74.
Hernández Centeno JR, Fernández Galicia JC, González Bravo F, Ramírez Barba EJ, Zavala Martín J, Montiel Ramírez AE, et al. Inicio temprano de la alimentación enteral en pacientes con reconexión intestinal. Nutr Clin y Diet Hosp. 2013;33:18–22.
Reddy V, Raghuramulu N, Bhaskaram C. Secretory IgA in protein-calorie malnutrition. Arch Dis Child. 1976;51:871–4.
Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr, Metab Immune Disord - Drug Targets. 2019;19:1100–15.
Kudsk KA, Hermsen JL, Genton L, Faucher L, Gomez FE. Injury stimulates an innate respiratory immunoglobulin a immune response in humans. J Trauma - Inj Infect Crit Care. 2008;64:316–23.
Sano Y, Hermsen JL, Kang W, Gomez FE, Lan J, Maeshima Y, et al. Parenteral nutrition maintains pulmonary IgA antibody transport capacity, but not active transport, following injury. Am J Surg. 2009;198:105–9.
Kondrup J, Ramussen HH, Hamberg O, Stanga Z, Camilo M, Richardson R, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–36.
Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15:R268.
The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N. Engl J Med. 1991;325:525–32.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
GQO, AESZ, and GGS were responsible for the main idea. GQO conducted the search of the studies and extract the information. GQO, MFRP, JGPC, AESZ, and GGS wrote the manuscript and designed the figure and table, and AESZ, GGS, and JGPC reviewed the final script.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quiroz-Olguín, G., Gutiérrez-Salmeán, G., Posadas-Calleja, J.G. et al. The effect of enteral stimulation on the immune response of the intestinal mucosa and its application in nutritional support. Eur J Clin Nutr 75, 1533–1539 (2021). https://doi.org/10.1038/s41430-021-00877-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-021-00877-7
This article is cited by
-
Nomogram to predict feeding intolerance in critically ill children
European Journal of Pediatrics (2023)