Abstract
Background/objectives
Western diet is characterized by a high acid load that could generate various degrees of metabolic acidosis, of which at least the stronger forms are known to contribute to the progression of chronic kidney disease (CKD). The aim of this study was to estimate the potential renal acid load (PRAL) and acid base status in CKD patients attended at the Children’s Hospital J.M. de los Ríos in Caracas, Venezuela from April 2015 to February 2016.
Subjects/methods
Twenty-seven children with CKD were included. Diet composition was evaluated by a food frequency questionnaire and a 24-h intake reminder. PRAL was calculated by the Remer and Manz method. Laboratory tests included serum creatinine, electrolytes and venous gases.
Results
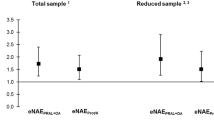
Protein intake was above recommendations in 21 patients (78.6%). Average vegetable and fruit intake was 0.4 and 1.5 servings per day, respectively. Mean PRAL was 16 ± 10.7 mEq/day. PRAL correlated positively with energy (p = 0.005), protein (p = 0.001) and fat intake (p = 0.0001), daily servings of dairy (p = 0.04) meat (p = 0.001) and cereals (0.001) and negatively with vegetable intake (p = 0.04). Serum pH and bicarbonate were 7.3 ± 0.08 and 20.46 ± 4.5 mEq/L, respectively. Twenty-one patients (80.7%) with metabolic acidosis were treated with sodium bicarbonate.
Conclusions
Dietary pattern of Venezuelan children with CKD may constitute a risk factor for the progression of the disease by promoting metabolic acidosis via unfavorable dietary acid loads. PRAL should be assessed as a valuable guide for nutritional counseling in children with CKD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Banerjee T, Liu Y, Crews DC. Dietary patterns and CKD progression. Blood Purif. 2016;41:117–22.
Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Ríos-Burrows N, et al. High Dietary Acid Load Predicts ESRD among Adults with CKD. J Am Soc Nephrol. 2015;26:1693–1700.
Goraya N, Wesson DE. Acid-base status and progression of chronic kidney disease. Curr Opin Nephrol Hypertens. 2012;21:552–6.
Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 2008;73:192–9.
Wesson D. Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int. 2012;82:1184–94.
Rebholz CM, Coresh J, Grams ME, Steffen LM, Anderson CA, Appel LJ, et al. Dietary acid load and incident chronic kidney disease: results from the ARIC study. Am J Nephrol. 2015;42:427–35.
Mirmiran P, Yuzbashian E, Bahadoran Z, Asghari G, Azizi F. Dietary acid-base load and risk of chronic kidney disease in adults. IJKD. 2016;10:119–25.
Krupp D, Esche J, Mensink GB, Klenow S, Thamm M, Remer T. Dietary acid load and potassium intake associate with blood pressure and hypertension prevalence in a representative sample of the German adult population. Nutrients. 2018;10:103. https://doi.org/10.3390/nu10010103. http://www.mdpi.com/2072-6643/10/1/103/htm.
Williams RS, Heilbronn LK, Chen DL, Coster AC, Greenfield JR, Samocha-Bonet D. Dietary acid load, metabolic acidosis and insulin resistance—lessons from cross-sectional and overfeeding studies in humans. Clin Nutr. 2015;35:1084–90.
Kiefte-de Jong JC, Li Y, Chen M, Curhan GC, Mattei J, Malik VSal. Diet-dependent acid load and type 2 diabetes: pooled results from three prospective cohort studies. Diabetologia 2017;60:270–9.
Han E, Kim G, Hong N, Lee Y, Kim DW, Shin HJ, et al. Association between dietary acid load and the risk of cardiovascular disease: nationwide surveys (KNHANES 2008–2011). Cardiovasc Diabetol. 2016;15:122
Banerjee T, Tucker K, Griswold M, Wyatt SB, Harman J, Young B, et al. Dietary potential renal acid load and risk of albuminuria and reduced kidney function in the Jackson Heart Study. J Ren Nutr. 2018;28:251–8.
Frassetto LA, Morris RC, Sellmeyer DE, Todd K, Sebastian A. Diet, evolution and aging. The pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur J Nutr. 2001;40:200–13.
Cordain L, Eaton SB, Sebastian A. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54.
Frassetto LA, Todd KM, Morris RC Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68:576–83.
Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59:1356–61.
Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95:791–7.
Harambat J, Kunzmann K, Azukaitis K, Bayazit AK, Canpolat N, Doyon A, et al. 4C Study Consortium. Metabolic acidosis is common and associates with disease progression in children with chronic kidney disease. Kidney Int. 2017;92:1507–14.
International Society of Nephrology. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO. Clinical Practice Int. 2012;Suppl.3:1–150.
Santos C, López de Martínez E Apéndice 4A: Lista de Intercambio de Alimentos. En: Dini E, Henríquez G, (editors). Nutrición en Pediatría. 2nd ed. Empresas Polar: Caracas, Venezuela; 2009. p. 1397–407.
National Kidney Foundation. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Am J Kidney Dis. 2009;53(Suppl. 2):S1–S124.
Instituto Nacional de Nutrición (INN). Guías de Alimentación para la población pediátrica. Caracas: INN; 1999.
National Research Council (NRC). Subcommittee on the Tenth Edition of the RDAs. Recommended Dietary Allowances. Washington: National Academy Press; 1989. p. 52–77.
Weiner JS, Lourie SA. Practical Human Biology. London: GB: Academy Press; 1981.
López-Blanco M, Macias-Tomei C, Espinoza-Izaguirre I, Colmenares R. Indice de masa corporal en niños del Estudio Longitudinal de Caracas. Ven Nutr. 1991;4:37–44.
World Health Organization AnthroPlus for personal computers manual: software for assessing growth of the world’s children and adolescents. 2011. http://www.who.int/growthref/tools/en/. Accessed 10 Feb 2017.
Schwartz GJ. Clinical assessment of renal function. In: Clinical Pediatric Nephrology, 2nd Ed., Kher KK, Schnaper HW, Makker SP Eds. Informa Healthcare: Abingdon, Oxon, UK; 2006. p. 71–93.
López-Sayers M, Bernal J, López M. Dietary potential renal acid load in Venezuelan children. Nutr Hosp. 2015;31:2054–61. https://doi.org/10.3305/nh.2015.31.5.818.
Alexy U, Kersting M, Remer T. Potential renal acid load in the diet of children and adolescents: impact of food groups, age and time trends. Public Health Nutr. 2008;11:300–6.
Torres-Cárdenas M, Méndez B, Landaeta-Jiménez M, Vázquez-Ramírez M. Consumo de alimentos y estado nutricional según estrato socioeconómico en una población infantil de Caracas. Arch Venez Puer Ped. 2011;74:54–61.
Nolan K, Schell LM, Stark A, Gómez MI. Longitudinal study of energy and nutrient intakes for infants from low-income, urban families. Public Health Nutr. 2002;5:405–12.
Moreno G, Campos I. Crecimiento y estado nutricional en niños con enfermedad renal crónica. Arch Venez Puer Pediatr. 2011;74:68–74.
Goraya N, Wesson DE. Dietary management of chronic kidney disease: protein restriction and beyond. Curr Opin Nephrol Hypertens. 2012;21:635–40.
Moe SM, Zidehsarai MP, Chambers MA. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–64.
Ingulli EG, Mak RH. Growth in children with chronic kidney disease: role of nutrition, growth hormone, dialysis, and steroids. Curr Opin Pediatr. 2014;26:187–92.
Kraut JA, Madias NE. Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr Nephrol. 2011;26:19–28.
Rodig NM, McDermott KC, Schneider MC, Hotchkiss HM, Yadin O, Furth SL, et al. Growth in children with chronic kidney disease: a report from the chronic kidney disease in children study. Pediatr Nephrol. 2014;29:1987–95.
Menon V, Tighiouart H, Vaughn NS. Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis. 2010;56:907–14.
Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transpl. 2009;24:1232–7.
Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011;79:356–62.
De Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–84.
Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol. 2012;35:540–7.
Gaggl M, Sliber C, Sunder-Plassmann G. Effect of oral alkali supplementation on progression of chronic kidney disease. Curr Hypertens Rev. 2014;10:112–20.
Dobre M, Rahman M, Hostetter TH. Current status of bicarbonate in CKD. J Am Soc Nephrol. 2015;26:515–23.
Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8:371–81.
Goraya N, Wesson DE. Dietary interventions to improve outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24:505–10.
Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86:1031–8.
Acknowledgements
No financial assistance was received in support of this study. This article is published as part of a supplement sponsored by NuOmix-Research K.S. The conference was financially supported by Protina Pharmazeutische GmbH, Germany and Sirius Pharma, Germany, and organized by NuOmix-Research K.S. Neither company had any role in writing of the paper.
Author information
Authors and Affiliations
Contributions
ML conceived and designed the study, participated in analysis and interpretation of data, and wrote the paper. G Moreno, GL, and G Marcano participated in the acquisition, analysis, and interpretation of data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
The study was approved by the institution’s Bioethics Committee. Children’s parents were informed in detail about the objectives of the research. A written consent was obtained from parents or representatives and from children over 8 years of age by means of an informed or assent consent, respectively.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
López, M., Moreno, G., Lugo, G. et al. Dietary acid load in children with chronic kidney disease. Eur J Clin Nutr 74 (Suppl 1), 57–62 (2020). https://doi.org/10.1038/s41430-020-0687-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-020-0687-3
This article is cited by
-
A Therapeutic Approach in the Management of Chronic Kidney Disease: Plant-Based Dietary Models and Associated Parameters
Current Nutrition Reports (2024)
-
Dietary acid load in children with chronic kidney disease: its association with nutritional status and health-related quality of life
Pediatric Nephrology (2023)
-
Scoping review of the dietary intake of children with chronic kidney disease
Pediatric Nephrology (2022)