Abstract
Background/objective
The relationship between postprandial glycaemic responses and cognitive performance, mood and satiety are inconsistent. The objective of this study is to compare the effects of different glycaemic responses, induced by beverages with different glycaemic index (GI) (sucrose and isomaltulose), and a non-glycaemic control (sucralose), on cognition, mood and satiety.
Subjects/methods
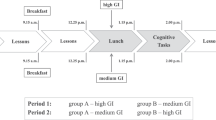
In this double-blinded, randomised crossover trial, healthy adults (n = 55) received sucrose (GI 65), isomaltulose (GI 32) and sucralose (non-caloric negative control) drinks on separate occasions. The Complex Figure test, the Word Recall test, Trail Making Test Part B and the Stroop test were administered 60 min after beverages ingestion. Mood and satiety were tested along with cognitive performance.
Results
Comparing between isomaltulose and sucrose, there were no significant differences in the mean (95% CI) for the following: Complex Figure: immediate recall −0.6 (−1.7, 0.5), delayed recall −0.8 (−1.9, 0.3); Word recall: immediate recall 0.2 (−0.7, 1.1), delayed recall 0.5 (−0.4, 1.4); Trail Making: completing time −2.4 (−7.5, 2.7) s; Stroop: time used for correct congruent responses −9 (−31, 14) ms and correct incongruent responses −18 (−42, 6) ms. No differences among beverages were found in the mood and satiety scores with exception that participants felt more energetic 60 min after isomaltulose ingestion (p = 0.028 for difference with sucrose) and hungrier 30 min after isomaltulose ingestion (p = 0.036 for difference with sucrose; p = 0.022 for difference with sucralose).
Conclusion
Under these study conditions there is no convincing evidence for an effect of glycaemic response on cognitive performance, mood or satiety.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–97.
Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25.
Patching SG. Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol. 2017;54:1046–77.
Messier C. Glucose improvement of memory: a review. Eur J Pharmacol. 2004;490:33–57.
Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, et al. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2002;22:271–9.
Meeusen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Brain neurotransmitters in fatigue and overtraining. Appl Physiol, Nutr, Metab. 2007;32:857–64.
Schneider F, Gur RE, Mozley LH, Smith RJ, Mozley PD, Censits DM, et al. Mood effects on limbic blood flow correlate with emotional self-rating: a PET study with oxygen-15 labeled water. Psychiatry Res. 1995;61:265–83.
Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37:811–23.
Chapman IM, Goble EA, Wittert GA, Morley JE, Horowitz M. Effect of intravenous glucose and euglycemic insulin infusions on short-term appetite and food intake. Am J Physiol. 1998;274:R596–R603.
Orzi F, Lucignani G, Dow-Edwards D, Namba H, Nehlig A, Patlak CS, et al. Local cerebral glucose utilization in controlled graded levels of hyperglycemia in the conscious rat. J Cereb Blood Flow Metab. 1988;8:346–56.
Pop MG, Crivii C, Opincariu I. Anatomy and function of the hypothalamus. IntechOpen. 2018;1:1–14.
Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–92.
Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci USA. 1996;93:4693–8.
Cooper SB, Bandelow S, Nute ML, Morris JG, Nevill ME. Breakfast glycaemic index and cognitive function in adolescent school children. Br J Nutr. 2012;107:1823–32.
Defeyter MA, Russo R. The effect of breakfast cereal consumption on adolescents’ cognitive performance and mood. Front Hum Neurosci. 2013;7:789.
Micha R, Rogers PJ, Nelson M. Glycaemic index and glycaemic load of breakfast predict cognitive function and mood in school children: a randomised controlled trial. Br J Nutr. 2011;106:1552–61.
Ingwersen J, Defeyter MA, Kennedy DO, Wesnes KA, Scholey AB. A low glycaemic index breakfast cereal preferentially prevents children’s cognitive performance from declining throughout the morning. Appetite. 2007;49:240–4.
LaCombe A, Ganji V. Influence of two breakfast meals differing in glycemic load on satiety, hunger, and energy intake in preschool children. Nutr J. 2010;9:53.
Kaplan RJ, Greenwood CE, Winocur G, Wolever TM. Dietary protein, carbohydrate, and fat enhance memory performance in the healthy elderly. Am J Clin Nutr. 2001;74:687–93.
Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr. 2012;108 Suppl 2:S105–12.
Lina BA, Jonker D, Kozianowski G. Isomaltulose (Palatinose): a review of biological and toxicological studies. Food Chem Toxicol. 2002;40:1375–81.
Park JY, Jung JH, Seo DH, Ha SJ, Yoon JW, Kim YC, et al. Microbial production of palatinose through extracellular expression of a sucrose isomerase from Enterobacter sp. FMB-1 in Lactococcus lactis MG1363. Bioresour Technol. 2010;101:8828–33.
Holub I, Gostner A, Theis S, Nosek L, Kudlich T, Melcher R, et al. Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose). Br J Nutr. 2010;103:1730–7.
The University of Sydney. https://www.glycemicindex.com/foodSearch.php. Accessed 28 May 2020.
Ginieis R, Franz EA, Oey I, Peng M. The “sweet” effect: comparative assessments of dietary sugars on cognitive performance. Physiol Behav. 2018;184:242–7.
Keesing C, Mills B, Rapsey C, Haszard J, Venn B. Cognitive performance following ingestion of glucose-fructose sweeteners that impart different postprandial glycaemic responses: a randomised control trial. Nutrients 2019;11:2647.
Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique.(Les problems.). Archives de Psychologie. 1941;28:215–85.
Strauss ShermanEMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press; 2006.
Brandt J. The Hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin Neuropsychologist. 1991;5:125–42.
Reitan RM. The relation of the trail making test to organic brain damage. J Consulting Psychol. 1955;19:393–4.
Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643.
Stoet G. Stroop task internet. https://www.psytoolkit.org/experiment-library/stroop.html. psytoolkit.org, United Kingdom. Accessed 4 June 2020.
Schmidt JR, De Houwer J. Now you see it, now you don’t: controlling for contingencies and stimulus repetitions eliminates the Gratton effect. Acta Psychologica. 2011;138:176–86.
Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–8.
Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord: J Int Assoc Study Obes. 2000;24:38–48.
Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9:1079–87.
Allen JB, Gross AM, Aloia MS, Billingsley C. The effects of glucose on nonmemory cognitive functioning in the elderly. Neuropsychologia. 1996;34:459–65.
Meikle A, Riby LM, Stollery B. The impact of glucose ingestion and gluco-regulatory control on cognitive performance: a comparison of younger and middle aged adults. Hum Psychopharmacol. 2004;19:523–35.
Sanchez-Aguadero N, Recio-Rodriguez JI, Patino-Alonso MC, Mora-Simon S, Alonso-Dominguez R, Sanchez-Salgado B, et al. Postprandial effects of breakfast glycaemic index on cognitive performance among young, healthy adults: A crossover clinical trial. Nutritional Neurosci. 2020;23:1–7.
Veasey RC, Gonzalez JT, Kennedy DO, Haskell CF, Stevenson EJ. Breakfast consumption and exercise interact to affect cognitive performance and mood later in the day. A randomized controlled trial. Appetite. 2013;68:38–44.
Brindal E, Baird D, Danthiir V, Wilson C, Bowen J, Slater A, et al. Ingesting breakfast meals of different glycaemic load does not alter cognition and satiety in children. Eur J Clin Nutr. 2012;66:1166–71.
Dye L, Gilsenan MB, Quadt F, Martens VEG, Bot A, Lasikiewicz N, et al. Manipulation of glycemic response with isomaltulose in a milk-based drink does not affect cognitive performance in healthy adults. Mol Nutr food Res. 2010;54:506–15.
Green MW, Taylor MA, Elliman NA, Rhodes O. Placebo expectancy effects in the relationship between glucose and cognition. Br J Nutr. 2001;86:173–9.
Schmitt JAJ, Benton D, Kallus KW. General methodological considerations for the assessment of nutritional influences on human cognitive functions. Eur J Nutr. 2005;44:459–64.
Philippou E, Constantinou M. The influence of glycemic index on cognitive functioning: a systematic review of the evidence. Adv Nutr. 2014;5:119–30.
Riby LM, McLaughlin J, Riby DM, Graham C. Lifestyle, glucose regulation and the cognitive effects of glucose load in middle-aged adults. Br J Nutr. 2008;100:1128–34.
van de Rest O, van der Zwaluw NL, de Groot LCPGM. Effects of glucose and sucrose on mood: a systematic review of interventional studies. Nutr Rev. 2018;76:108–16.
Shlomowitz A, Feher MD. Anxiety associated with self monitoring of capillary blood glucose. Br J Diabetes Vasc Dis. 2014;14:60–3.
Nichol AD, Holle MJ, An R. Glycemic impact of non-nutritive sweeteners: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2018;72:796–804.
Zanchi D, Meyer-Gerspach AC, Schmidt A, Suenderhauf C, Depoorter A, Drewe J, et al. Acute effects of glucose and fructose administration on the neural correlates of cognitive functioning in healthy subjects: a pilot study. Front Psychiatry. 2018;9:71.
Acknowledgements
The authors would like to thank Mr Meng Tong who designed the software and website used in the cognitive test.
Funding
This study was funded by the University of Otago.
Author information
Authors and Affiliations
Contributions
BJV conceived and supervised the study. BJV and QD designed and undertook the glycaemic testing. CR, QD and MP designed the cognitive testing. TSC was responsible for designing and overseeing the mood component. BJV designed the satiety testing. JJH was responsible for the statistical approach and for undertaking the statistical analysis of the cognitive, mood and satiety data. MP analysed the results of sensory tests. QD undertook the practical work and drafted the manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Q., Haszard, J.J., Conner, T.S. et al. Cognitive performance, mood and satiety following ingestion of beverages imparting different glycaemic responses: a randomised double-blind crossover trial. Eur J Clin Nutr 75, 602–610 (2021). https://doi.org/10.1038/s41430-020-00749-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-020-00749-6