Abstract
Background
Aflatoxins are found in diverse foods widely consumed worldwide. This study investigated the association between aflatoxin exposure and (a) consumption of specific foods, (b) dietary diversity (DD), and (c) seasonality.
Methods
Women enrolled in the AflaCohort Study in Banke, Nepal (n = 1648) were asked how often they ate certain food items in the past 7 days and 24 h. Serum aflatoxin B1-lysine (AFB1-lys) adduct levels, measured during pregnancy, were determined using high-performance liquid chromatography. Multivariable ordinary least squares and quantile regression models were used to examine incremental increases in AFB1-lys adduct levels per frequency of food consumption and the relationship between DD, seasonality, and increases in AFB1-lys adduct.
Results
Roughly 94% of women were exposed to aflatoxin (geometric mean 1.37 pg/mg). Women in the 30th, 50th, and 70th quantiles of aflatoxin exposure who reported one more occasion of maize consumption in the past week showed increases in AFB1-lys adduct levels: 0.094, 0.112, and 0.109 pg/mg (p < 0.05, all). Women in the 30th, 50th, 70th, and 90th quantiles of exposure who reported one more occasion of groundnut consumption in the past week also showed increases in AFB1-lys adduct levels: 0.058 (p < 0.001), 0.085 (p < 0.01), 0.133 (p < 0.001), and 0.133 (p < 0.001) pg/mg. Winter month recruitment was positively associated with AFB1-lys adduct levels at all quantiles of aflatoxin exposure (range: 0.313–1.101 pg/mg, p < 0.001). DD was not predictive of aflatoxin exposure.
Conclusions
Our findings justify integrated approaches to aflatoxin reduction, including regulatory, agricultural, and food safety interventions across the value chain and at the household level.
Similar content being viewed by others
Introduction
In South Asia, women and young children are at risk of exposure to aflatoxin, a naturally occurring toxin produced by Aspergillus fungi [1, 2]. Acute aflatoxicosis can cause coma or death. Chronic, low level exposure to aflatoxin is harmful to human health [3]. Evidence shows placental transfer of aflatoxin from mother to fetus [4] and linkages to impaired linear growth in childhood [4, 5].
Exposure occurs primarily through the consumption of contaminated foods. Maize, chilies, spices, oilseeds, and nuts are especially susceptible to aflatoxin contamination [6,7,8]. When ruminants ingest feed contaminated with aflatoxin they metabolize and excrete the metabolite, aflatoxin M1 (AFM1), in milk [9]. Aflatoxins are difficult to detect and remove because they are unobservable to the consumer and relatively resistant to thermal inactivation [10, 11].
Populations at particularly high risk of chronic aflatoxin exposure are resource-scarce, have limited dietary variety, store foods for long periods, and rely on highly susceptible foods including maize and groundnuts [12, 13]. Access to improved dietary diversity (DD) may lower aflatoxin exposure by lessening the dependence on aflatoxin-prone foods and counteracting the toxicity [14, 15], particularly in those consuming monotonous diets [16, 17].
This study was conducted to determine: (a) if the frequency of consumption of susceptible agricultural commodities was associated with aflatoxin exposure in pregnancy in Nepali women, (b) if increased DD was associated with lower levels of aflatoxin exposure, and (c) whether aflatoxin exposure levels vary seasonally.
Methods
Study population
The AflaCohort Birth Cohort Study (2015–2019) was conducted in Banke, a tropical district (Province 5) in the southern plains of Nepal. A rolling recruitment strategy was used to enroll 1675 healthy pregnant women. The sample size was calculated assuming an alpha of 0.05, power of 80%, attrition of 20%, and design effect of 1.5. This allowed the detection of a −0.207 standard deviations (SD) difference in postnatal height-for-age Z-score for every 1-unit increase in log average maternal AFB1-lys adducts.
Eligibility criteria included: <30 weeks pregnant, age 16–49, singleton pregnancy, living, and planning to give birth in the study area. This analysis used data collected during pregnancy (July 2015–August 2016); nation-wide strikes interrupted data collection for 3 months and resumed in December 2015.
The women (or their legal guardians) gave verbal and written consent prior to participation. The Nepal Health Research Council (295/2014), and the Tufts Institutional Review Board (11535) approved this study.
Data collection
Trained interviewers administered electronic surveys. Surveys included a single qualitative 7 and 24 h food frequency questionnaire (FFQ) [18] to determine the frequency of consumption of 49 predetermined food items. The food items included were based on previous dietary assessments in this population [19]. Consumption data were also collected for the past year.
Upon survey completion, interviewers measured height, weight and mid-upper arm circumference (MUAC) to the nearest 0.1 cm and 0.1 kg using ShorrBoard® Measuring Boards, 874 Seca Scales, and 65 cm adult measuring tapes, respectively.
Within a week of survey completion, nurses visited the women and collected a 3–5 mL antecubital vein blood sample. Blood samples were transported on wet ice to a local laboratory for processing. Samples were air-shipped on wet ice to the Patan Academy of Health Sciences to be stored at −80 °C until they were ready to be air-shipped on dry ice to the Wang laboratory at the University of Georgia.
Data analysis
A total of 1650 gestational serum samples were analyzed for AFB1-lys adducts, an established biomarker of dietary aflatoxin exposure over the previous 2–3 months [20]. The levels of AFB1-lys adducts were measured using a validated high-performance liquid chromatography (HPLC) with fluorescence detection method [21].
After deactivation in 56 °C water bath for 30 min, ~150 μL of each sample were digested by pronase (pronase: total protein, 1:4, w/w) at 37 °C for 3 h to release adducts. The digests were extracted and purified by passing through a Waters MAX SPE cartridge, eluted with 2% formic acid in methanol, vacuum-dried with a Labconco Centrivap concentrator (Kansas City, MO), and reconstituted with 25% methanol water for HPLC-fluorescence detection.
An Agilent 1200 HPLC-fluorescence system (Santa Clara, CA) was used to quantify AFB1-lys adducts. The mobile phases consisted of buffer A (20 mM NH4H2PO4, pH 7.2) and buffer B (100% Methanol), running at a gradient to allow separation within 25 min of injection, with a typical retention time for AFB1-lys adduct at ~13 min. Separation was achieved using Zorbax Eclipse XDB-C18 reverse phase column (5 micron, 4.6 × 250 mm) equipped with a guard column, maintained at 25 °C and a flow rate of 1 mL/min during analysis. Sample injection volume was 100 μL. Excitation and emission wavelengths for detection were 405 and 470 nm, respectively. Calibration curves of authentic standard were generated weekly. Quality assurance and quality control procedures included simultaneous analysis of one authentic standard for every ten samples, and two daily quality control samples. The average recovery rate was 90% for the report, the AFB1-lys concentration was adjusted by albumin concentration, measured via UV/Visible spectrophotometry. Samples below the limit of detection (LOD) (0.4 pg AFB1-lysine/mg albumin) were substituted with a constant value of half the LOD for statistical analysis [22].
Minimum DD scores were computed using Minimum Dietary Diversity for Women of Reproductive Age (MDD-W) guidelines [23]. Food items from the 24-h FFQ were categorized into one of ten food groups: [1] grains/roots/tubers, [2] pulses, [3] nuts and seeds, [4] dairy, [5] meats, [6] eggs, [7] dark green leafy vegetables (DGLV), [8] other vitamin A sources, [9] other vegetables, [10] other fruits. A dichotomous MDD indicator was created to calculate whether women achieved MDD (consuming ≥ 5 of the 10 food groups in the previous 24 h).
Covariates analyzed included age, education, wealth status, MUAC, season, and Village Development Committee with those included in the models being selected based on their potential for confounding.
Seasonal variations in aflatoxin exposure were examined for autumn, prewinter, winter, spring, summer, and monsoon seasons. Autumn is characterized by wet, cool weather, while the prewinter and winter are cooler and drier [24]. Spring and summer are warm and dry. The summer months are the hottest while the rainy/monsoons are the most humid. Annual rainfall during the survey period (mean = 1232 mm, SD = 577) did not differ significantly (t = 0.12) from prior years—January 1999–December 2014 (mean = 1263, SD = 468) with no differences in monthly averages between the two periods [25].
Principal component analysis [26,27,28,29] was used to construct a composite measure for household wealth. Data on type of roof, floor, walls, toilet, cooking fuel, piped water, number of household members, and asset ownership (livestock, radio, television, mobile phone, bicycle, motorcycle, electric fan) were used to construct the variable.
Data were divided into quintiles of aflatoxin B1-lys adducts (lowest: ≤0.5823; low: >0.5825 to ≤0.9219; middle: >0.9219 to ≤1.4322; high: >1.422 to ≤2.9315; very high: >2.9315 pg/mg). Nonnormally distributed AFB1-lys data were natural log-transformed for all statistical analyses.
Two-sided Student’s t tests and analysis of variance and chi-squared tests tested continuous variables and dichotomous variables, respectively. Covariate-adjusted parameter estimates with 95% confidence intervals were computed using ordinary least squares (OLS) and quantile regression (QR) [30]. QR models were used to quantify the associations of aflatoxin-prone-food consumption frequency and maternal DD at different points of the aflatoxin distribution (10th, 30th, 50th, 70th, and 90th quantiles). Restricted cubic splines and nonparametric smoothing curves helped test for an unadjusted nonlinear relationships [31]. Variance inflation factors helped diagnose multicollinearity among the predictor variables in the regression models. Significance levels were set at p < 0.05. Statistical analyses were conducted using Stata 14.2 (StataCorp LP).
Results
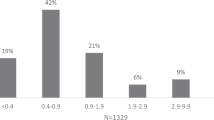
AFB1-lys adducts (range: 0.4–147 pg/mg albumin) were found in 94% of samples (Fig. 1; mean concentration 3.2 ± 8.3 pg/mg albumin, geometric mean 1.37 pg/mg albumin, CI: 1.3–1.4).
In the past 24 h, 100% women consumed rice, 62% consumed pulses, while 12% consumed nuts/seeds (Fig. 2). Dairy was more commonly consumed than meat (43% versus 25%) in the past 24 h. While over 80% reported consuming vegetables, the reported 24-h consumption of fruit, eggs, DGLV, and vitamin A-rich sources was low (33%, 12%, 28%, and 20%, respectively). Only 39% achieved MDD.
Age and MUAC were significantly negatively associated with maternal AFB1-lys adduct concentrations in bivariate analyses. Hemoglobin level and winter season were significantly positively associated with aflatoxin exposure (Table 1).
Weekly consumption frequencies included 31% reporting consumption of groundnuts, 3% reporting maize, and 2% reporting both groundnuts and maize (Table 2). The weekly mean frequency of maize and groundnuts consumption was 2.4 ± 2.2 and 2.8 ± 2.2, respectively. AFB1-lys adduct levels were significantly higher in maize and groundnut consumers (7.9 pg/mg albumin adducts) compared with those who did not eat maize or groundnuts (2.4 pg/mg albumin adducts, p < 0.0001) in the past week.
Weekly maize consumption did not vary across wealth quintiles and was positively associated with maternal education (p < 0.01) (Table 3). Weekly groundnut consumption, in contrast, was positively associated with wealth status (p < 0.01) but not with maternal education. Frequency of maize (p < 0.05) and groundnut (p < 0.001) consumption in the past week were significantly higher for women recruited in the winter.
Annual consumption frequencies included 83% reporting maize, 97% groundnut, 100% chilies, and 93% reporting consuming milk (Table 2). No association was detected between aflatoxin exposure and annual consumption of maize or groundnuts. Low variability in annual chili consumption limited our ability to test the association with aflatoxin exposure. Annual milk consumption was positively associated with AFB1-lys adduct concentrations (p < 0.05). Neither annual wheat nor rice consumption was associated with maternal aflatoxin exposure (data not shown).
In the adjusted OLS model, groundnut consumption in the past week (0.730, p < 0.001) and the winter season (2.339, p < 0.001) were significant predictors of maternal AFB1-lys adduct levels (Table 4). In the QR models, maize and groundnut consumption were heterogeneously positively associated with higher aflatoxin. Every additional occasion of reported weekly maize consumption was associated with higher AFB1-lys adduct concentrations in the 30th (0.094, p < 0.05), 50th (0.112, p < 0.05), and 70th quantiles (0.109, p < 0.05) of exposure. In contrast, reported weekly maize consumption was not associated with aflatoxin exposure in the OLS regression or in the QR model in the 10th and 90th quantiles of exposure.
Women in the 30th, 50th, and 70th quantiles of exposure who reported one more occasion of weekly groundnut consumption experienced significantly higher aflatoxin levels: 0.058 (p < 0.001), 0.085 (p < 0.01), and 0.133 (p < 0.001) pg AFB1-lys adducts per mg of albumin. Similarly, women in the 90th quantile of exposure reporting one more occasion of weekly groundnut consumption showed significantly higher concentrations of AFB1-lys adduct (0.133, p < 0.001). However, weekly groundnut consumption was not associated with aflatoxin for women in the 10th quantile of exposure; this may be a function of lower groundnut consumption in women with the lowest aflatoxin levels. Restricted cubic spline analyses found no evidence of a threshold effect between either weekly maize or groundnut consumption and exposure. This suggests that a linear relationship hypothesis between weekly consumption and exposure cannot be rejected for this sample, i.e., frequent consumption results in higher values in blood.
Women in the 10th and 50th quantiles of exposure who reported milk consumption had higher aflatoxin exposure (0.63 (p < 0.01) and 0.23 (p < 0.05), respectively) than those who did not consume milk in the past year. DD was not associated with maternal aflatoxin exposure in the OLS or at most quantiles in the QR models. DD scores were significantly positively associated with maternal aflatoxin in the 10th quantile of exposure (0.064, p < 0.05). The association between winter season and AFB1-lys adduct concentration was positive across all quantiles.
Discussion
Biomarker data show that the majority of the women were exposed to aflatoxin during pregnancy. Diet-associated aflatoxin exposure in these women seems to be driven by groundnut and maize consumption and is highly variable by season of measurement. Contrary to expectations, results showed no association between DD and maternal aflatoxin levels.
The geometric mean of serum maternal AFB1-lysine adduct concentration of 1.37 pg/mg albumin (95% CI: 1.30, 1.44 pg/mg albumin) in this cohort was lower than average concentrations found in similar studies. One Nepali study [1] reported 3.62 pg AFB1-lysine/mg albumin (geometric mean) in children ages 15–36 months, while two other studies in African children [32, 33] reported levels ranging 4.5–8.3 pg/mg.
The positive associations between weekly maize and groundnut consumption and serum AFB1-lys adduct concentrations are consistent with previous research as contamination is common in these commodities [34,35,36,37]. Maize and groundnut products have been known to commonly exceed the permissible limit for aflatoxin [34, 38,39,40]. While maize and groundnut production is low in the Banke area these two foods seem to be important sources of aflatoxin exposure.
Groundnuts are a nutrient-dense food, high in protein, fats, fiber, and multiple micronutrients and are a common snack in Nepal. They have recently gained popularity through government promotion programs [41]. Commercialization of groundnut products and market trends present an opportunity for spreading awareness and targeted measures to improve the quality of groundnut and groundnut products. Awareness campaigns and aflatoxin reduction interventions can help reduce consumption of aflatoxin-contaminated foods without compromising demand for nutrient-dense food items.
Aflatoxin M1, a hydroxylated metabolite of AFB1, can be found in milk or milk products from livestock that have ingested contaminated feed. Although it was beyond the scope of this study to measure AFM1, our study did examine the association between consuming milk and serum AFB1-lysine adduct concentration levels. Our findings showing positive associations between milk consumption during the past year and increased aflatoxin levels are in line with Kafle et al. [42] showing 44% of milk samples contaminated with aflatoxin M1. Indirect sources of contamination such as milk should not be overlooked when designing aflatoxin reduction interventions.
Although this study did not find an association between rice consumption and aflatoxin levels, rice cannot be disregarded as it is a fundamental component of the Nepali diet and can harbor low levels of aflatoxin [43]. Future work should also examine other commonly contaminated, ubiquitous foods and spices, such as black pepper, nutmeg, cumin, coriander, garlic, and dairy products (e.g., curd) [44].
Previous research suggests that DD reduces the amount of aflatoxin-prone foods consumed and counteracts adverse effects of aflatoxin [16]. Our study, with a population reliant on rice, found no association between higher DD and lower aflatoxin exposure. Findings suggest that those who diversified their diets with groundnuts or maize increased their exposure to aflatoxins. Nevertheless, DD promotion, which brings important benefits, should continue in nutrition interventions. Focused actions to lower contamination risk in these two foods should be prioritized in nutrition strategies designed to promote DD.
Seasonal variations in serum aflatoxin levels were apparent in this study, with the highest levels of exposure seen during the dry, cool winter. This strong association between AFB1-lysine adduct concentrations and winter season is consistent with the previous literature [2, 45,46,47]. Higher consumption of contaminated foods can come from either increased quantity consumed after harvest and/or consumption of lower quality, more contaminated foods that had been stored for long periods of time in either the household or market. Maize and groundnuts are typically harvested between August and September when optimum conditions for Aspergillus growth prevail. Prolonged, multimonth postharvest storage and suboptimal drying and storage conditions in hot, humid areas can lead to increased aflatoxin production during winter.
This study was the first to measure the association of maize and groundnut consumption and DD with maternal aflatoxin levels in pregnant women in Nepal. Findings can be used to plan interventions aimed at lowering exposure to aflatoxin, particularly in vulnerable populations. The results are generalizable because the large sample size reflected the communities represented and women were sampled from varied sociodemographic and economic circumstances. Furthermore, the outcome variable, maternal AFB1-lys adduct concentration, was objectively measured using HPLC. The use of QR in the analysis was an important methodological contribution not found in previous research, which has mostly relied on OLS and logistic regression. Unlike OLS, QR does not assume normality or homoscedasticity of errors and is much less influenced by extreme values of serum AFB1-lys. QR produced a more nuanced picture of the effects of maize and groundnut consumption patterns and DD on maternal aflatoxin exposure.
Our study has limitations. Some of the variation observed in AFB1-lys may be explained by factors we did not account for (e.g., quantities consumed, quality of the aflatoxin-prone foods consumed, food preparation methods, or individual variation in overall xenobiotic loads) [48]. Second, the study did not measure consumption over the previous 2–3 month period that is characteristic of AFB1-lys adduct half-life in the body. Finally, due to the rolling nature of the recruitment process aflatoxin data during autumn months were not available.
Results confirmed widespread aflatoxin exposure in pregnancy and showed that consumption of maize and/or groundnut consumption are dietary contributors of aflatoxin even in areas with rice-based diets. Our findings strongly support further consideration of targeted regulatory, agricultural, and food safety interventions across the value chain and at the household level to reduce aflatoxin exposure. Aflatoxin reduction campaigns should inform pregnant women and their families of both the nutritional value of consuming maize and groundnuts and of the special precautions that should be taken when purchasing, storing, and consuming agricultural food items susceptible to aflatoxin contamination. A combination of proven practical, low-cost aflatoxin reduction techniques (e.g., removal of contaminated kernels) at the household level and market level regulation of aflatoxin-prone foods could help reduce exposure to aflatoxin in vulnerable populations.
References
Mitchell NJ, Hsu HH, Chandyo RK, Shrestha B, Bodhidatta L, Tu YK, et al. Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: an extension of the MAL-ED study. PLoS ONE. 2017;12:e0172124.
Groopman JD, Egner PA, Schulze KJ, Wu LS, Merrill R, Mehra S, et al. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B(1)-lysine albumin biomarkers. Food Chem Toxicol. 2014;74:184–9.
Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82.
Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41:740–55.
Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, et al. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ health Perspect. 2004;112:1334–8.
Kimanya ME, De Meulenaer B, Tiisekwa B, Ndomondo-Sigonda M, Devlieghere F, Van Camp J, et al. Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Addit Contam Part A Chem Anal Control Exposure Risk Assess. 2008;25:1353–64.
Leong YH, Rosma A, Latiff AA, Izzah AN. Associations of serum aflatoxin B1-lysine adduct level with socio-demographic factors and aflatoxins intake from nuts and related nut products in Malaysia. Int J Hyg Environ Health. 2012;215:368–72.
Lombard MJ. Mycotoxin exposure and infant and young child growth in Africa: what do we know? Ann Nutr Metab. 2014;64 Suppl 2 :42–52.
Prandini A, Tansini G, Sigolo S, Filippi L, Laporta M, Piva G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem Toxicol. 2009;47:984–91.
Moser CM, Hoffmann V. Firm heterogeneity in food safety provision: evidence from aflatoxin tests in Kenya. Washington, DC: International Food Policy Research Institute (IFPRI). 2013. https://ssrn.com/abstract=2567751.
Magan N, Olsen M. Mycotoxins in feed detection and control, part II controlling risks. Cambridge, United Kingdom: Woodhead Publishing; 2004.
Turner PC. The molecular epidemiology of chronic aflatoxin driven impaired child growth. Scientifica: Hindawi Publishing Corporation 2013;2013:1–21.
Turner PC, Flannery B, Isitt C, Ali M, Pestka J. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr Res Rev. 2012;25:162–79.
Groopman JD, Kensler TW, Wild CP. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu Rev Public Health. 2008;29:187–203.
Chen JG, Egner PA, Ng D, Jacobson LP, Munoz A, Zhu YR, et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev Res. 2013;6:1038–45.
Wu F, Mitchell NJ, Male D, Kensler TW. Reduced foodborne toxin exposure is a benefit of improving dietary diversity. Toxicological Sci. 2014;141:329–34.
Schwartzbord J, Brown D, Pape J, Verdier R, Filbert M, Wang JS. Aflatoxin–lysine adducts in haitian patients ingesting peanut and maize products. J Hunger Environ Nutr. 2014;9:244–55.
Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health 2014;36:e2014009.
Campbell RK, Talegawkar SA, Christian P, Leclerq SC, Khatry SK, Wu LS, et al. Evaluation of a novel single-administration food frequency questionnaire for assessing seasonally varied dietary patterns among women in rural Nepal. Ecol Food Nutr. 2015;54:314–27.
Vettorazzi A, López de Cerain A. Chapter 17—Mycotoxins as food carcinogens. In: Carla Viegas CP, Raquel Sabino, Susana Viegas, João Brandão, Cristina Veríssimo, editor. Environmental Mycology in Public Health. United Stated of America: Academic Press; 2016. p. 261–98.
Qian G, Tang L, Wang F, Guo X, Massey ME, Williams JH, et al. Physiologically based toxicokinetics of serum aflatoxin B1-lysine adduct in F344 rats. Toxicology. 2013;303:147–51.
Jin Y, Hein MJ, Deddens JA, Hines CJ. Analysis of lognormally distributed exposure data with repeated measures and values below the limit of detection using SAS. Ann Occup Hyg. 2011;55:97–112.
FAO/FHI 360. Minimum dietary diversity for women: a guide to measurement. Rome, Italy: FAO; 2016.
Government of Nepal. Banke district profile. Government of Nepal: 2017; Kathmandu, Nepal. http://www.namis.gov.np/downloadfile/Banke District Profile_SKM_1433381537.pdf.
Nepal Government Department of Hydrology and Meteorology Monthly Rainfall Data (1999–2018). Ministry of Energy Water Resources and Irrigation. Kathmandu, Nepal. 2018. http://moewri.gov.np/en/.
Rutstein SO. Steps to constructing the new DHS Wealth Index. 2016. Rockville, MD: ICF International https://dhsprogram.com/programming/wealth index/Steps_to_constructing_the_new_DHS_Wealth_Index.pdf.
Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–68.
Filmer D, Pritchett LH. Estimating wealth effects without expenditure data-or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–32.
Ministry of Health—Nepal, New Era, ICF. Nepal demographic and health survey 2011. Kathmandu, Nepal: Ministry of Health; 2012.
Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62:511–7 e1.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–57.
Hoffmann V, Jones K, Leroy JL. The impact of reducing dietary aflatoxin exposure on child linear growth: a cluster randomised controlled trial in Kenya. BMJ Glob Health. 2018;3:e000983.
Lauer JM, Duggan CP, Ausman LM, Griffiths JK, Webb P, Wang JS, et al. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern Child Nutr. 2019;15:e12701.
Egal S, Hounsa A, Gong YY, Turner PC, Wild CP, Hall AJ, et al. Dietary exposure to aflatoxin from maize and groundnut in young children from Benin and Togo, West Africa. Int J Food Microbiol. 2005;104:215–24.
Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–22.
Koirala P, Kumar S, Yadav BK, Premarajan KC. Occurrence of aflatoxin in some of the food and feed in Nepal. Indian J Med Sci. 2005;59:331–6.
Shirima CP, Kimanya ME, Kinabo JL, Routledge MN, Srey C, Wild CP, et al. Dietary exposure to aflatoxin and fumonisin among Tanzanian children as determined using biomarkers of exposure. Mol Nutr food Res. 2013;57:1874–81.
Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, et al. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. BMJ. 2002;325:20–1.
Shuaib FM, Jolly PE, Ehiri JE, Ellis WO, Yatich NJ, Funkhouser E, et al. Socio-demographic determinants of aflatoxin B1-lysine adduct levels among pregnant women in Kumasi, Ghana. Ghana Med J. 2012;46:179–88.
Turner PC, Collinson AC, Cheung YB, Gong Y, Hall AJ, Prentice AM, et al. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol. 2007;36:1119–25.
Kattel S, Chhetri R, Dhakal S. Peanut cultivation and consumption in Nepal: a social cultural perspective. Kathmanud, Nepal: Tribhuban University; 2002.
Kafle P, Sedai D, Rai KP, Pokharel BB. Study on the level of aflatoxin M1 contamination in raw and processed milk marketed in Kathmandu Valley. J Food Sci Technol Nepal. 2012;7:52–56.
Elzupir AO, Alamer AS, Dutton MF. The occurrence of aflatoxin in rice worldwide: a review. Toxin Rev. 2015;34:37–42.
Hammami W, Fiori S, Al Thani R, Ali Kali N, Balmas V, Migheli Q, et al. Fungal and aflatoxin contamination of marketed spices. Food Control. 2014;37:177–81.
Castelino JM, Dominguez-Salas P, Routledge MN, Prentice AM, Moore SE, Hennig BJ, et al. Seasonal and gestation stage associated differences in aflatoxin exposure in pregnant Gambian women. Tropical Med Int Health. 2014;19:348–54.
Wild CP. Aflatoxin exposure in developing countries: the critical interface of agriculture and health. Food Nutr Bull. 2007;28 2 Suppl :S372–80.
Wild CP, Yin F, Turner PC, Chemin I, Chapot B, Mendy M, et al. Environmental and genetic determinants of aflatoxin-albumin adducts in the Gambia. Int J Cancer. 2000;86:1–7.
Dellafiora L, Dall'Asta C. Forthcoming challenges in mycotoxins toxicology research for safer food-a need for multi-omics approach. Toxins. 2017;9:1–14
Acknowledgements
The authors express special gratitude to USAID-Nepal Mission, USAID Bureau of Food Security, Dr Maura Mack, Dr Ahmed Kablan, Mr Debendra Adhikari, Nepalgunj Medical College, AflaCohort study team, and the study participants without whom this research would not have been possible.
Funding
Support for this research was provided by the Feed the Future Innovation Lab for Nutrition, which is funded by the United States Agency for International Development (USAID) under grant ID: AID-OAA-L-10-00006. The opinions expressed herein are solely those of the authors.
Author information
Authors and Affiliations
Contributions
JYA-T contributed to study design and was responsible for analysis and manuscript writing. PW and SG were responsible for the overall design, planning, and contributed to the statistical analysis and writing of the paper. KB was responsible for the overall design and planning of the study. GS and BLR contributed to the statistical analysis of the study, interpretation and contributed to the writing of the paper. DD, KP, and RS contributed to the design and implemented the study. AP supervised the fieldwork. JW and KSX conducted the analysis of aflatoxin-exposure markers. All authors reviewed the manuscript for accuracy and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andrews-Trevino, J.Y., Webb, P., Shively, G. et al. Dietary determinants of aflatoxin B1-lysine adduct in pregnant women consuming a rice-dominated diet in Nepal. Eur J Clin Nutr 74, 732–740 (2020). https://doi.org/10.1038/s41430-019-0554-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0554-2
This article is cited by
-
Dietary determinants of aflatoxin B1-lysine adduct among infants in Nepal
European Journal of Clinical Nutrition (2022)
-
Evaluation of acute and chronic exposure to aflatoxin B1 in indigenous women of the Huasteca Potosina, Mexico
Environmental Science and Pollution Research (2020)