Abstract
Background/objectives
The objective of this study was to investigate the efficacy of small-quantity lipid-based nutrient supplements (SQ-LNS) containing essential fatty acids (EFAs) with or without long-chain polyunsaturated fatty acids (LCPUFAs) in improving LCPUFA status in South African infants fed complementary food.
Subjects/methods
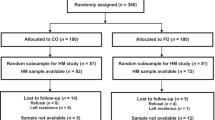
Six-month-old infants (n = 750) were randomised to receive SQ-LNS, SQ-LNS-plus, or no supplement. Both SQ-LNSs contained micronutrients and EFAs. SQ-LNS-plus additionally contained the LCPUFAs arachidonic acid (AA) and docosahexaenoic acid (DHA), lysine, phytase and other nutrients. Plasma total phospholipid FA composition (% of total FAs) was measured at baseline (n = 353) and at 12 months (n = 293).
Results
At baseline, geometric mean (95% CI) plasma DHA and AA were 4.1 (4.0–4.3) and 11.5 (11.2–11.8)% respectively, with significantly higher plasma DHA and AA in breastfed than non-breastfed infants. Infants receiving the SQ-LNS-plus had significantly higher plasma DHA (4.52 (4.3–4.9)) at 12 months than the controls (3.8 (3.6–4.0)), with a higher effect size in infants who no longer received breast milk (β = 1.148 (95% CI = 0.597, 1.699)) than in infants who were still breastfeeding (β = 0.544 (95% CI = 0.179, 0.909)). There was no effect of either of the two SQ-LNSs on plasma AA. Consequently, infants receiving the SQ-LNS-plus had a significantly lower plasma n-6 to n-3 PUFA ratio at 12 months than control infants did.
Conclusions
Our study suggests that the provision of SQ-LNS-plus is efficacious in improving plasma DHA status. Particularly, infants who are no longer breastfed may benefit most from LCPUFA-enriched SQ-LNS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Carlson SE, Colombo J. Docosahexaenoic acid and arachidonic acid nutrition in early development. Adv Pediatr 2016;63:453–71.
Innis SM. Fatty acids and early human development. Early Hum Dev 2007;83:761–6.
Koletzko B, Lien E, Agostoni C, Böhles H, Campoy C, Cetin I, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med [Internet]. 2008;36:5–14.
World Health Organization (WHO), United Nations Children Fund (UNICEF). Global strategy for infant and young child feeding. World Health Organization; 2003.
Libuda L, Mesch CM, Stimming M, Demmelmair H, Koletzko B, Warschburger P, et al. Fatty acid supply with complementary foods and LC-PUFA status in healthy infants: results of a randomised controlled trial. Eur J Nutr 2016;55:1633–44.
Michaelsen KF, Hoppe C, Roos N, Kaestel P, Stougaard M, Lauritzen L, et al. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr Bull 2009;30:S343–404.
EFSA Panel on Dietetic Products N and A (NDA). Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J 2013;11:3408.
Forsyth S, Gautier S, Salem JrN. Estimated dietary intakes of arachidonic sacid and docosahexaenoic acid in infants and young Children living in developing countries. Ann Nutr Metab 2016;69:64–74.
Michaelsen KF, Dewey KG, Perez-Exposito AB, Nurhasan M, Lauritzen L, Roos N. Food sources intake n-6 n-3 Fat acids low-income countries Emphas infants, Young- child (6-24 Mon), pregnant Lactating women. Matern child Nutr 2011;7:124–40.
Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 2013;382:452–77.
Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr 2007;86:412–20.
Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, et al. Complementary feeding with fortified spread and incidence of severe stunting in 6-to 18-month-old rural Malawians. Arch Pediatr Adolesc Med 2008;162:619–26.
Prado EL, Abbeddou S, Yakes Jimenez E, Somé JW, Ouédraogo ZP, Vosti SA, et al. Lipid-based nutrient supplements plus malaria and diarrhea treatment increase infant development scores in a cluster-randomized trial in Burkina Faso. J Nutr 2015;146:814–22.
Prado EL, Maleta K, Ashorn P, Ashorn U, Vosti SA, Sadalaki J, et al. Effects of maternal and child lipid-based nutrient supplements on infant development: a randomized trial in Malawi. Am J Clin Nutr 2016;103:784–93.
Matias SL, Mridha MK, Tofail F, Arnold CD, Khan MSA, Siddiqui Z, et al. Home fortification during the first 1000 d improves child development in Bangladesh: a cluster-randomized effectiveness trial. Am J Clin Nutr 2017;105:958–69.
Smuts CM, Matsungo TM, Malan L, Kruger HS, Rothman M, Kvalsvig, JD, et al. Effect of small-quantity lipid-based nutrient supplements on growth, psychomotor development, iron status, and morbidity among 6- to 12-mo-old infants in South Africa: a randomized controlled trial. Ameri J Clin Nutr 2019;109:55–68.
Rothman M, Berti C, Smuts CM, Faber M, Covic N. Acceptability of novel small-quantity lipid-based nutrient supplements for complementary feeding in a peri-urban south african communityy. Food Nutr Bull 2015;36:455–66.
World health Organisation. Physical Status: The Use and Interpretation of Anthropometry. ‘Report of a WHO expert committee’. World Health Organ Tech Rep Ser 1995;854:1–452.
World Health Organization (WHO). WHO child growth standards: length/height for age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age, methods and development. World Health Organization; 2006.
Siziba LP, Baumgartner J, Ricci C, Jacobs A, Rothman M, Matsungo TM, et al. Associations of plasma total phospholipid fatty acid patterns with feeding practices, growth and psychomotor development in six-month-old South African infants. Matern Child Nutr 2018;15:e12763.
Makrides M, Hawkes JS, Neumann MA, Gibson RA. Nutritional effect of including egg yolk in the weaning diet of breast-fed and formula-fed infants: a randomized controlled trial. Am J Clin Nutr 2002;75:1084–92.
Hoffman DR, Birch EE, Castañeda YS, Fawcett SL, Wheaton DH, Birch DG, et al. Visual function in breast-fed term infants weaned to formula with or without long-chain polyunsaturates at 4 to 6 months: a randomized clinical trial. J Pediatr 2003;142:669–77.
Hoffman DR, Theuer RC, Castañeda YS, Wheaton DH, Bosworth RG, O’Connor AR, et al. Maturation of visual acuity is accelerated in breast-fed term infants fed baby food containing DHA-enriched egg yolk. J Nutr 2004;134:2307–13.
Grote V, Verduci E, Vecchi F, Contarini G, Giovannini M, Koletzko B, et al. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur J Clin Nutr 2016;70:250–6.
Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 2007;85:1457–64.
Brenna JT. Arachidonic acid needed in infant formula when docosahexaenoic acid is present. Nutr Rev 2016;74:329–36.
Hadley K, Ryan A, Forsyth S, Gautier S, Salem N. The essentiality of arachidonic acid in infant development. Nutrients 2016;8:216.
Forsyth JS. Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: follow up of a randomised controlled trial. BMJ 2003;326:953–953.
Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Llong-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res 2011;70:406–10.
Forsyth S, Gautier S, Salem N. The importance of dietary DHA and ARA in early life: a public health perspective. Proc Nutr Soc 2017;76:568–73.
Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr 2014;99:734S–41S.
Gibson RA, Neumann MA, Lien EL, Boyd KA, Tu WC. Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. Prostaglandins, Leukot Essent Fat Acids 2013;88:139–46.
Hess SY, Abbeddou S, Jimenez EY, Somé JW, Vosti SA, Ouédraogo ZP, et al. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young Burkinabe children: a cluster-randomized trial. PloS ONE 2015;10:e0122242.
Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clin Biochem 2010;43:338–40.
Harris WS, Varvel SA, Pottala JV, Warnick GR, McConnell JP. Comparative effects of an acute dose of fish oil on omega-3 fatty acid levels in red blood cells versus plasma: implications for clinical utility. J Clin Lipidol 2013;7:433–40.
Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n–3 fatty acid content of blood cells and plasma phospholipids–. Am J Clin Nutr 2007;86:1621–5.
Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem 2006;52:2265–72.
Acknowledgements
We extend our utmost gratitude to all the caregivers and infants who participated in the study as well as all the fieldworkers for their hard work and dedication to the study. We also thank nurses Chrissie Lessing and Linda Lemmer for their invaluable clinical expertise as well as the laboratory team of the Centre of Excellence for Nutrition for their support and lab assistance.
Funding
Global Alliance for Improved Nutrition (GAIN), Geneva, Switzerland, funded the main Tswaka study. DSM & UNILEVER were co-funders. UNILEVER R&D Vlaardingen B.V. (Vlaardingen, The Netherlands) provided the SQ-LNS product. DSM Nutritional products Ltd supplied the SQ-LNS-plus.
Author information
Authors and Affiliations
Contributions
CMS, JB and MF conceptualised and designed the study; CMS was the principal investigator of the Tswaka study and had overall responsibility for data collection. MF was the co-principal investigator of the Tswaka study, contributed to the study design and provided guidance on analysis of feeding practices. MR and TMM were the study co-ordinators of the Tswaka study and helped to execute the study. MR, TMM and LPS executed the study and collected data. LPS quantified the plasma phospholipid FAs. LPS and JB performed statistical analyses; CMS and JB provided guidance on statistical analysis and interpretation. LPS wrote the first draft of the manuscript and all authors read, revised and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
CMS received travel support from Unilever, DSM and Sight and Life; TMM received a speaking honorarium from DSM; LPS received travel support from DSM and Sight and Life. However, the principal investigator (CMS) made final decisions on the interpretation and dissemination of results. None of the other researchers has any conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siziba, L.P., Baumgartner, J., Rothman, M. et al. Efficacy of novel small-quantity lipid-based nutrient supplements in improving long-chain polyunsaturated fatty acid status of South African infants: a randomised controlled trial. Eur J Clin Nutr 74, 193–202 (2020). https://doi.org/10.1038/s41430-019-0482-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0482-1