Abstract
Background/Objectives
To identify factors associated with plasma polyunsaturated fatty acid (PUFA) levels among 3-month-old Tanzanian infants.
Subjects/Methods
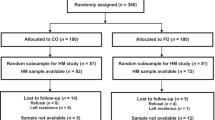
Infants (n = 238) and mothers (n = 193) randomly selected from participants in the neonatal vitamin A supplementation randomized controlled trial. A cross-sectional study of maternal–infant pairs at 3 months postpartum.
Results
All infant total, n-3, n-6, and individual PUFA levels were correlated with maternal levels. Infant plasma n-3 PUFA levels were higher when maternal n-3 PUFA levels were higher (mean difference in infant % fatty acid per unit increase in maternal levels ± standard error: 0.79 ± 0.08; P < 0.01). Infant plasma docosahexaenoic acid (DHA) levels were positively associated with maternal DHA levels (0.77 ± 0.09; P < 0.01) but were lower for twin births (−0.55 ± 0.27; P = 0.03). Greater birth weight in kilograms (1.00 ± 0.43; P = 0.02) and higher maternal n-6 PUFA levels (0.20 ± 0.07; P < 0.01) were positively associated with higher infant n-6 PUFA levels, whereas maternal mono-unsaturated fatty acid (MUFA) levels (−0.26 ± 0.08; P < 0.01), maternal mid upper arm circumference (MUAC) (−0.22 ± 0.11; P = 0.04), and male sex (−0.99 ± 0.45; P = 0.03) were associated with lower infant plasma n-6 PUFA levels. Infant plasma arachidonic acid (AA) levels were positively associated with maternal plasma AA levels (0.38 ± 0.09; P < 0.01), but inversely associated with twin births (−1.37 ± 0.67; P = 0.04).
Conclusions
Greater birth weight and higher maternal plasma PUFA levels at 3 months postpartum were significantly associated with higher infant plasma PUFA levels at 3 months age. Twin births, male sex, and higher maternal MUFA levels were associated with lower infant plasma PUFA levels. Nutrition counseling for optimal intake of PUFA-rich foods, to lactating mothers in resource-limited settings may be beneficial for improved infant health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Babirekere-Iriso E, Mortensen CG, Mupere E, Rytter MJ, Namusoke H, Michaelsen KF, et al. Changes in whole-blood PUFA and their predictors during recovery from severe acute malnutrition. Br J Nutr. 2016;115:1730–9.
Warner JO. The early life origins of asthma and related allergic disorders. Arch Dis Child. 2004;89:97–102.
Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83:1337–44.
Wyrwoll CS, Mark PJ, Mori TA, Puddey IB, Waddell BJ. Prevention of programmed hyperleptinemia and hypertension by postnatal dietary omega-3 fatty acids. Endocrinology. 2006;147:599–606.
Enke U, Seyfarth L, Schleussner E, Markert UR. Impact of PUFA on early immune and fetal development. Br J Nutr. 2008;100:1158–68.
Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94.
Calder PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. 2012;56:1073–80.
Berghaus TM, Demmelmair H, Koletzko B. Essential fatty acids and their long-chain polyunsaturated metabolites in maternal and cord plasma triglycerides during late gestation. Biol Neonate. 2000;77:96–100.
Innis SM. Essential fatty acids in growth and development. Prog Lipid Res. 1991;30:39–103.
Arbuckle LD, Innis SM. Docosahexaenoic acid is transferred through maternal diet to milk and to tissues of natural milk-fed piglets. J Nutr. 1993;123:1668–75.
De Giuseppe R, Roggi C, Cena H. n-3 LC-PUFA supplementation: effects on infant and maternal outcomes. Eur J Nutr. 2014;53:1147–54.
Brenna JT. Arachidonic acid needed in infant formula when docosahexaenoic acid is present. Nutr Rev. 2016;74:329–36.
da Costa Rde S, Santos Fda S, da Conceicao FD, Saunders C, Sardinha FL, Chaves CR, et al. Plasma levels of trans-fatty acids are low in exclusively breastfed infants of adolescent mothers. Lipids. 2011;46:537–43.
Olsen SF, Hansen HS, Sorensen TI, Jensen B, Secher NJ, Sommer S, et al. Intake of marine fat, rich in (n-3)-polyunsaturated fatty acids, may increase birthweight by prolonging gestation. Lancet (Lond, Engl). 1986;2:367–9.
Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol. 2003;101:469–79.
Morse NL. Benefits of docosahexaenoic acid, folic acid, vitamin D and iodine on foetal and infant brain development and function following maternal supplementation during pregnancy and lactation. Nutrients. 2012;4:799–840.
Makrides M, Neumann MA, Gibson RA. Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. Eur J Clin Nutr. 1996;50:352–7.
SanGiovanni JP, Berkey CS, Dwyer JT, Colditz GA. Dietary essential fatty acids, long-chain polyunsaturated fatty acids, and visual resolution acuity in healthy fullterm infants: a systematic review. Early Hum Dev. 2000;57:165–88.
Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot Essent Fat Acids. 2009;81:151–8.
Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–67.
Fewtrell MS, Abbott RA, Kennedy K, Singhal A, Morley R, Caine E, et al. Randomized, double-blind trial of long-chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. J Pediatr. 2004;144:471–9.
Kannass KN, Colombo J, Carlson SE. Maternal DHA levels and toddler free-play attention. Dev Neuropsychol. 2009;34:159–74.
Mozurkewich EL, Klemens C. Omega-3 fatty acids and pregnancy: current implications for practice. Curr Opin Obstet Gynecol. 2012;24:72–7.
Furuhjelm C, Warstedt K, Fageras M, Falth-Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505–14.
Kremmyda LS, Vlachava M, Noakes PS, Diaper ND, Miles EA, Calder PC. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: a systematic review. Clin Rev Allergy Immunol. 2011;41:36–66.
Pastor N, Soler B, Mitmesser SH, Ferguson P, Lifschitz C. Infants fed docosahexaenoic acid- and arachidonic acid-supplemented formula have decreased incidence of bronchiolitis/bronchitis the first year of life. Clin Pediatr. 2006;45:850–5.
Birch EE, Khoury JC, Berseth CL, Castaneda YS, Couch JM, Bean J, et al. The impact of early nutrition on incidence of allergic manifestations and common respiratory illnesses in children. J Pediatr. 2010;156:902–6, 6e1.
Minns LM, Kerling EH, Neely MR, Sullivan DK, Wampler JL, Harris CL, et al. Toddler formula supplemented with docosahexaenoic acid (DHA) improves DHA status and respiratory health in a randomized, double-blind, controlled trial of US children less than 3 years of age. Prostaglandins Leukot Essent Fat Acids. 2010;82:287–93.
Field CJ, Thomson CA, Van Aerde JE, Parrott A, Euler A, Lien E, et al. Lower proportion of CD45R0 + cells and deficient interleukin-10 production by formula-fed infants, compared with human-fed, is corrected with supplementation of long-chain polyunsaturated fatty acids. J Pediatr Gastroenterol Nutr. 2000;31:291–9.
Group NSA, Bahl R, Bhandari N, Dube B, Edmond K, Fawzi W, et al. Efficacy of early neonatal vitamin A supplementation in reducing mortality during infancy in Ghana, India and Tanzania: study protocol for a randomized controlled trial. Trials. 2012;13:22.
Masanja H, Smith ER, Muhihi A, Briegleb C, Mshamu S, Ruben J, et al. Effect of neonatal vitamin A supplementation on mortality in infants in Tanzania (Neovita): a randomised, double-blind, placebo-controlled trial. Lancet (Lond, Engl). 2015;385:1324–32.
Kabagambe EK, Baylin A, Allan DA, Siles X, Spiegelman D, Campos H. Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long-term dietary intake. Am J Epidemiol. 2001;154:1126–35.
Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36.
Miettinen OS. Theoretical epidemiology: principles of occurrence research in medicine. New York, USA: John Wiley & Sons Inc.; 1985.
Jumbe T, Comstock SS, Hahn SL, Harris WS, Kinabo J, Fenton JI. Whole blood levels of the n-6 essential fatty acid linoleic acid are inversely associated with stunting in 2-to-6 year old Tanzanian children: a cross-sectional study. PLoS ONE. 2016;11:e0154715.
Hurtado JA, Iznaola C, Pena M, Ruiz J, Pena-Quintana L, Kajarabille N, et al. Effects of maternal omega-3 supplementation on fatty acids and on visual and cognitive development. J Pediatr Gastroenterol Nutr. 2015;61:472–80.
Peng YM, Zhang TY, Wang Q, Zetterstrom R, Strandvik B. Fatty acid composition in breast milk and serum phospholipids of healthy term Chinese infants during first 6 weeks of life. Acta Paediatr (Oslo, Nor: 1992). 2007;96:1640–5.
Gibson RA, Neumann MA, Makrides M. Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. Eur J Clin Nutr. 1997;51:578–84.
Fares S, Sethom MM, Kacem S, Khouaja-Mokrani C, Feki M, Kaabachi N. Plasma arachidonic and docosahexaenoic acids in Tunisian very low birth weight infants: status and association with selected neonatal morbidities. J Health Popul Nutr. 2015;33:1.
van der Merwe LF, Moore SE, Fulford AJ, Halliday KE, Drammeh S, Young S, et al. Long-chain PUFA supplementation in rural African infants: a randomized controlled trial of effects on gut integrity, growth, and cognitive development. Am J Clin Nutr. 2013;97:45–57.
Elias SL, Innis SM. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr. 2001;73:807–14.
Uauy R, Mena P, Wegher B, Nieto S, Salem N Jr. Long chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatr Res. 2000;47:127–35.
Montgomery C, Speake BK, Cameron A, Sattar N, Weaver LT. Maternal docosahexaenoic acid supplementation and fetal accretion. Br J Nutr. 2003;90:135–45.
Henderson RA, Jensen RG, Lammi-Keefe CJ, Ferris AM, Dardick KR. Effect of fish oil on the fatty acid composition of human milk and maternal and infant erythrocytes. Lipids. 1992;27:863–9.
Makrides M, Neumann M, Simmer K, Pater J, Gibson R. Are long-chain polyunsaturated fatty acids essential nutrients in infancy? Lancet (Lond, Engl). 1995;345:1463–8.
Goodnight W, Newman R. Society of Maternal-Fetal M Optimal nutrition for improved twin pregnancy outcome. Obstet Gynecol. 2009;114:1121–34.
Yildiz A, Balikci E, Gurdogan F. Serum mineral levels at pregnancy and postpartum in single and twin pregnant sheep. Biol Trace Elem Res. 2005;107:247–54.
Foreman-van Drongelen MM, Zeijdner EE, van Houwelingen AC, Kester AD, Al MD, Hasaart TH, et al. Essential fatty acid status measured in umbilical vessel walls of infants born after a multiple pregnancy. Early Hum Dev. 1996;46:205–15.
McFadyen M, Farquharson J, Cockburn F. Maternal and umbilical cord erythrocyte omega-3 and omega-6 fatty acids and haemorheology in singleton and twin pregnancies. Arch Dis Child Fetal Neonatal Ed. 2003;88:F134–8.
Acknowledgements
We thank the mothers and their infants for participating voluntarily in this study and the communities where the primary trial was carried out for their support and cooperation. We thank the health authorities in the three municipals of Ilala, Kinondoni, and Temeke in Dar es Salaam, and Kilombero, Ulanga, and Kilosa districts in Morogoro region. We thank the coordinators, supervisors, research assistants, field supervisors, field interviewers, and health and demographic surveillance system staff at Ifakara Health Institute for their commitment during the implementation of the primary trial.
Funding
The parent trial was funded through a grant from the Bill and Melinda Gates Foundation to the World Health Organization (grant no: OPPGH5297). The funder had no role in the design, analysis, or writing of this article.
Author information
Authors and Affiliations
Contributions
PK: analysis plan, data analysis, interpretation of analysis results, and writing this article; HM and WWF: designed the original neonatal vitamin A (NEOVITA) trial, the present study being a secondary analysis of data collected in NEOVITA; EH, EKK and WWF: provided statistical guidance in data analysis; EH, EKK and WWF: provided guidance in interpretation of analysis results; ERS, AM, SM, CB and RAN: participated in field implementation of the primary trial; all authors have provided comments on earlier drafts of the paper and read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamenju, P., Hertzmark, E., Kabagambe, E.K. et al. Factors associated with plasma n-3 and n-6 polyunsaturated fatty acid levels in Tanzanian infants. Eur J Clin Nutr 74, 97–105 (2020). https://doi.org/10.1038/s41430-019-0428-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0428-7
This article is cited by
-
Maternal PUFAs, Placental Epigenetics, and Their Relevance to Fetal Growth and Brain Development
Reproductive Sciences (2023)