Abstract
Background/objectives
Recent data have shown that dividing skeletal muscle (SM) into sub-ranges of radiodensity can improve the prediction of short-term outcomes in the oncology setting. We aim to investigate whether the skeletal muscle mass, when divided into sub-ranges of low or high-radiodensity, improves the prediction of short-term survival in endometrial cancer (EC) patients when compared to average muscle attenuation and to the overall skeletal muscle radiodensity.
Subjects/methods
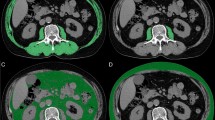
EC patients who had computed tomography (CT) images available within 30 days before treatment were enrolled in this retrospective cohort (n = 232). CT images at the third lumbar vertebra (L3) were used to assess overall skeletal muscle index (SMI). Then we divided SMI into sub-ranges of radiation attenuation: low-radiodensity skeletal muscle index (LRSMI) and high-radiodensity skeletal muscle index (HRSMI). The average muscle radiation attenuation was also assessed. Low SMI was defined when SMI was <38.9 cm2/m2. One-year survival was evaluated by Kaplan–Meier method and Cox Regression.
Results
Sarcopenia was found in 25.8% of the patients. Roughly 80% of the patients in the highest quartile of LRSMI were obese. All the skeletal muscle parameters were significantly associated with shorter 1-year survival, LRSMI presented a trend for significance in the adjusted model. When the SM parameters were additionally adjusted for low SMI, only HRSMI and LRSMI remained in the model as early-mortality predictors.
Conclusions
Classifying the skeletal muscle into sub-ranges of radiodensity have an additional value than using the average muscle attenuation of the overall skeletal muscle area and should be exploited in further studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Thibault R, Genton L, Pichard C. Body composition: why, when and for who? Clin Nutr. 2012;31:435–47.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67.
Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210:489–97.
Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35:1103–9.
Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–40.
Dériaz O, Dumont M, Bergeron N, Després J-P, Brochu M, Prud’homme D. Skeletal muscle low attenuation area and maximal fat oxidation rate during submaximal exercise in male obese individuals. Int J Obes. 2001;25:1579–84.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22.
Kaibori M, Ishizaki M, Iida H, Matsui K, Sakaguchi T, Inoue K, et al. Effect of intramuscular adipose tissue content on prognosis in patients undergoing hepatocellular carcinoma resection. J Gastrointest Surg. 2015;19:1315–23.
Malietzis G, Johns N, Al-Hassi HO, Knight SC, Kennedy RH, Fearon KCH, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016;263:320–5.
Silva de Paula N, de Aguiar Bruno K, Azevedo Aredes M, Villaça Chaves G. Sarcopenia and skeletal muscle quality as predictors of postoperative complication and early mortality in gynecologic cancer. Int J Gynecol Cancer. 2018;28:412–20.
Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4.
World Health Organization. Report of a WHO Expert Consultation: World Health Organization Technical Report, Series number 854. Physical status: the use and interpretation of anthropometry. 1995.
OPAS OPA. XXXVI Reunión Del Comitê Asesor de Investigaciones En Salud – Encuestra Multicêntrica – Salud Beinestar Y Envejecimeiento (SABE) En América Latina E El Caribe-Informe Preliminar. 2002.
Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006.
Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–99.
Rodrigues CS, Chaves GV. Patient-generated subjective global assessment in relation to site, stage of the illness, reason for hospital admission, and mortality in patients with gynecological tumors. Support Care Cancer. 2015;23:871–9.
Rodrigues CS, Lacerda MS, Chaves GV. Patient generated subjective global assessment as a prognosis tool in women with gynecologic cancer. Nutrition. 2015;31:1372–8.
Zoico E, Corzato F, Bambace C, Rossi AP, Micciolo R, Cinti S, et al. Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatr. 2013;57:411–6.
Maddocks M, Wilcock A. Exploring physical activity level in patients with thoracic cancer: implications for use as an outcome measure. Support Care Cancer. 2012;20:1113–6.
Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33.
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–7.
Barbat-Artigas S, Rolland Y, Vellas B, Aubertin-Leheudre M. Muscle quantity is not synonymous with muscle quality. J Am Med Dir Assoc. 2013;14:852. e1-7
Rodrigues CS, Chaves GV. Skeletal muscle quality beyond average muscle attenuation: a proposal of skeletal muscle phenotypes to predict short-term survival in patients with endometrial cancer. J Natl Compr Canc Netw. 2018;16:153–160.
Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94:2104–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
de Paula, N.S., Rodrigues, C.S. & Chaves, G.V. Comparison of the prognostic value of different skeletal muscle radiodensity parameters in endometrial cancer. Eur J Clin Nutr 73, 524–530 (2019). https://doi.org/10.1038/s41430-018-0163-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-018-0163-5
This article is cited by
-
Pre-treatment sarcopenic assessments as a prognostic factor for gynaecology cancer outcomes: systematic review and meta-analysis
European Journal of Clinical Nutrition (2022)