Abstract
Background/objectives
Cold exposure increases thermogenesis and could improve insulin sensitivity. We hypothesized a blunted response in the metabolic syndrome (MetS).
Subjects/methods
Twenty older adults 59 ± 10.4 years (with MetS, MetS+, n = 9; without MetS, MetS−, n = 11) completed a randomized crossover design of 3.5 h exposures to 20, 25 and 27 °C on three visits. After an hour’s rest at the desired temperature, resting metabolic rate (RMR), respiratory quotient (RQ), forearm to fingertip gradients (FFG), and in the ear temperature (IET) were measured over 30 min. An oral glucose tolerance test followed, and serial measurements were continued for 2 h. Venous blood was sampled for clinical chemistry, irisin, and fibroblast growth factor 21(FGF21). A mixed model ANCOVA adjusted data for age, gender, fat mass, fat-free mass and seasonality.
Results
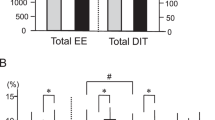
There was a significant MetS×temperature interaction where adjusted RMR was significantly higher in MetS+ compared to MetS− by 12% at 20 °C and by 6% at 25 °C, but similar at 27 °C. FFG increased and IET decreased with decreasing temperature to the same extent in both groups. Fasting irisin and FGF21 did not vary with temperature but the former was significantly higher in MetS−. Adjusted postprandial RQ and insulin to glucose ratios were significantly higher at 20 °C relative to 25 °C. Partial correlation analysis of differences between 27 and 20 °C indicated significant positive relationships between fasting as well as postprandial RQ and the respective changes in irisin and FGF21.
Conclusions
There could be an upward shift of the TNZ in MetS+, but this needs reevaluation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R285–96.
Cannon B, Nedergaard J. Brown adipose tissue:function and physiological significance. Physiol Rev. 2004;84:277–359.
Lowell B, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–60. https://doi.org/10.1038/35007527
Kingma B, Frijns AJ, Saris WH, van Steenhoven AA, van Marken Lichtenbelt WD, Increased systolic blood pressure after mild cold and rewarming: relation to cold-induced thermogenesis and age. Acta Physiol. 2011;203:419–27.
van der Lans AAAJ, Hoeks J, Brans B, Vijgen GHEJ, Visser MGW, Vosselman MJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–34038.
Van Pelt RE, Dinneno FA, Seals DR. Age-related decline in RMR in physically active men: relation to exercise volume and energy intake. Am J Physiol Endocrinol Metab. 2001;281:E633–9.
Wijers SLJ, Saris Wim HM, Van Marken Lichtenbelt WD. Cold-induced adaptive thermogenesis in lean and obese. Obesity. 2010;18:1092–9.
Brychta R, Chen KY. Cold-induced thermogenesis in humans. Eur J Clin Nutr. 2017;71:345–52.
Calton E, Soares MJ, James AP, Woodman RJ. The potential role of irisin in the thermoregulatory responses to mild cold exposure in adults. Am J Hum Bio. 2016;28:699–704.
Gisolfi C, Mora F. The hot brain: survival, temperature and the human body. Cambridge: MIT Press; 2000.
Lemieux H, Blier PU, Tardif JC. Does membrane fatty acid composition modulate mitochondrial functions and their thermal sensitivities? Comp Biochem Physiol A Mol Integr Physiol. 2008;149:20–9.
Chen K, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab. 2013;98:E1218–23. https://doi.org/10.1210/jc.2012-4213
Broeders E, Bouvy ND, van Marken Lichtenbelt WD, Endogenous ways to stimulate brown adipose tissue in humans. Ann Med. 2015;47:123–32.
Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9.
Daskalopoulou S, Cooke AB, Gomez YH, Mutter AF, Filippaios A, Mesfum ET, et al. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur J Endocrinol. 2014;171:343–52.
Choi H, Kim S, Park JW, Lee NS, Hwang SY, Huh J, et al. Implication of Circulating Irisin Levels with Brown Adipose Tissue and Sarcopenia in Humans. J Clin Endocrinol Metab. 2014;99:2778–85.
Fisher F, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1a and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–81.
Celi F, Butler PW, Brychta RJ, Linderman JD, Alberobello AT, Smith S, et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol. 2010;163:863–72.
Warwick PM, Busby R. Influence of mild cold on 24 h energy expenditure in ‘normally’ clothed adults. Br J Nutr. 1990;63:481–8.
Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–9.
Cameron A, Magliano DJ, Zimmet PZ, Welborn T, Shaw JE. The metabolic syndrome in Australia: prevalence using four definitions. Diabetes Res Clin Pract. 2007;77:471–8.
Waterhouse DF, McLaughlin AM, Sheehan F, O’Shea D. An examination of the prevalence of IDF- and ATPIII-defined metabolic syndrome in an Irish screening population. Ir J Med Sci. 2009;178:161–6.
Zhao Y, Yan H, Yang R, et al. Prevalence and determinants of metabolic syndrome among adults in a rural area of Northwest China. PLoS One. 2014;9:e91578.
Sawant A, Mankeshwar R, Shah S, Raghavan R, Dhongde G, Raje H, et al. Prevalence of metabolic syndrome in urban India. Cholesterol. 2011;2011:920983.
Bonet M, Pico C, Palou. A thermogenesis and the metabolic syndrome. Pathogenesis. Spain: University of The Balearic Islands: Laboratory of Molecular Biology, Nutrition And Biotechnology, Department of Fundamental Biology And Health Sciences; 2006, p. 284–303.
Pathak K, Soares MJ, Zhao Y, James AP, Sherriff JS, Newsholme P. Vitamin D status but not fibroblast growth factor 21 improved postprandial glucose oxidation and insulin sensitivity in the metabolic syndrome. Nutrition. 2017;37:37–42.
Novotny D, Vaverkova H, Karasek D, Lukes J, Slavik L, Malina P et al. Evaluation of total adiponectin, adipocyte fatty acid binding protein and fibroblast growth factor 21 levels in individuals with metabolic syndrome. Physiol Res. 2014;63:219–28.
Zhang X, Yeung DCY, Karpisek M, Stejskal D, Zhou Z, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–53.
Yan B, Shi X, Zhang H, Pan L, Ma Z, Liu S, et al. Association of serum irisin with metabolic syndrome in obese chinese adults. PLoS One. 2014;9:e94235.
Faul F, Erdfelder E, Lang AG, Buchner A, G*Power3: A flexible statistical power 290 analysis program for the social, behavioral, and biomedical sciences. Beh Res Methods. 2007;39:175–91.
Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Strobbe H, Schrauwen P. Energy metabolism in humans at a lowered ambient temperature. Eur J Clin Nutr. 2002;56:288–96.
Saghaei M, Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. https://doi.org/10.1186/1471-2288-4-26
Alberti K, Zimmet P, Shaw J. Metabolic Syndrome- a new world-wide definition. A consensus Statement from the International Diabetes Federation. Diab Med. 2006;23:469–80.
Hopkins WG. Calculations for reliability. A New View of Statistics. Internet Society for Sport Science. 2000. http://www.sportsci.org/resource/stats/relycalc.html
Kingma B, Frijns A, van Marken, Lichtenbelt WD, The thermoneutral zone: implications for metabolic studies. Front Biosci. 2012;4:1975–85.
Meigal A. Gross and fine neuromuscular performance at cold shivering. Int J Circumpolar Health. 2002;61:163–72.
DeGroot D, Kenney WL, Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol. 2007;292:R103–R108. https://doi.org/10.1152/ajpregu.00074.2006
Kingma B, Frijns AJH, Schellen L, van Marken Lichtenbelt WD. Beyond the classic thermoneutral zone: Including thermal comfort. Temp. 2014;1:142–9.
Park K, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98:4899–907.
Fukushima Y, Kurose S, Shinno H, Thu HC, Tamanoi A, Tsutsumi H, et al. Relationships between serum irisin levels and metabolic parameters in Japanese patients with obesity. Obes Sci Pract. 2016;2:203–9. https://doi.org/10.1002/osp4.43
Flatt J. Use and storage of carbohydrate and fat. Am J Clin Nutr. 1995;61:952S–995.
Dauncey M. Influence of mild cold on 24 h energy expenditure, resting metabolism and diet-induced thermogenesis. Br J Nutr. 1981;45:257.
Korwutthikulrangsri M, Poomthavorn P, Mahachoklertwattana P, Chanprasertyothin S, Khlairit P, Pongratanakul S. Serum fibroblast growth factor 21 in obese children and its dynamic changes during an oral glucose challenge. Chicago: Endocrine Society’s 96th Annual Meeting and Expo; 2014.
Lin Z, Gong Q, Wu C, Yu J, Lu T, Pan X et al. Dynamic change of serum FGF21 levels in response to glucose challenge in human. J Clin Endocrinol Metab. 2012;97:E1224–8.
Straczkowski M, Kowalska I, Nikolajuk A, Adamska A, Karczewska-Kupczewska M, Lebkowska A, et al. Serum fibroblast growth factor 21 in human obesity: regulation by insulin infusion and relationship with glucose and lipid oxidation. Int J Obes. 2013;37:1386–90.
van Marken Lichtenbelt WD, Kingma B, van der Lans A, Schellen L. Cold exposure – an approach to increasing energy expenditure in humans. Sci Soc. 2014;25:165–7.
Lorenzo C, Haffner SM, Stancˇa´ kova´ A, Laakso M. Relation of direct and surrogate measures of insulin resistance to cardiovascular risk factors in nondiabetic finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab. 2010;95:5082–90.
Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. Clin Invest. 2014;124:515–27. https://doi.org/10.1172/JCI67353
Lopez-Legarrea P, de la Iglesia R, Crujeiras AB, Pardo M, Casanueva FF, Zulet MA et al. Higher baseline irisin concentrations are associated with greater reductions in glycemia and insulinemia after weight loss in obese subjects. Nutr Diab. 2014;4:e110.
Semba R, Sun K, Ferrucci L. Relationship of serum fibroblast growth factor 21 with abnormal glucose metabolism and insulin resistance: the baltimore longitudinal study of aging. J Clin Endochrinol Metab. 2012;97:1375–82.
Graham TE. Thermal, metabolic, and cardiovascular changes in men and women during cold stress. Med Sci Sports Exerc. 1988;20:185–92.
Pettit S, Marchand I, Graham T. Gender differences in cardiovascular and catecholamine responses to cold-air exposure at rest. Can J Appl Physiol. 1999;24:131–47.
Childs C, Harrison R, Hodkinson C. Tympanic membrane temperature as a measure of core temperature. Arch Dis Child. 1999;80:262–6.
Chamberlain J, Terndrup TE, Alexander DT, Silverstone FA, Wolf-Klein G, O'Donnell R, et al. Determination of normal ear temperature with an infrared emission detection thermometer. Ann Emerg Med. 1995;25:15–20.
Casa D, Becker SM, Ganio MS, Brown CM, Yeargin SW, Roti MW, et al. Validity of devices that assess body temperature during outdoor exercise in the heat. J Athl Train. 2007;42:333–42.
Acknowledgements
M.J.S. acknowledges infrastructure support from the School of Public Health Curtin University. K.P. was the recipient of an Australian Postgraduate Award. We thank the reviewers for their insightful comments and attention to detail, which have helped shape this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pathak, K., Woodman, R.J., James, A.P. et al. Fasting and glucose induced thermogenesis in response to three ambient temperatures: a randomized crossover trial in the metabolic syndrome. Eur J Clin Nutr 72, 1421–1430 (2018). https://doi.org/10.1038/s41430-017-0058-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-017-0058-x
This article is cited by
-
Measurement of Energy Expenditure by Indirect Calorimetry with a Whole-Room Calorimeter
Phenomics (2024)
-
The evolving view of thermogenic fat and its implications in cancer and metabolic diseases
Signal Transduction and Targeted Therapy (2022)