Abstract
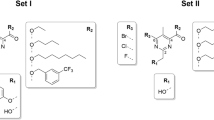
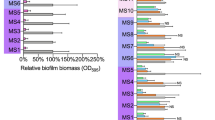
This study aims to explore the potential targets of bithionol in Staphylococcus aureus.The four bithionol biotinylated probes Bio-A2-1, Bio-A2-2, Bio-A2-3, and Bio-A2-4 were synthesized, the minimal inhibitory concentrations (MICs) of these probes against S. aureus were determined. The bithionol binding proteins in S. aureus were identified through immunoprecipitation and LC-MS/MS with bithionol biotinylated probe. The biotinylated bithionol probes Bio-A2-1 and Bio-A2-3 displayed antibacterial activities against S. aureus. The Bio-A2-1 showed lower MICs than Bio-A2-3, and both with the MIC50/MIC90 at 12.5/12.5 μM against S. aureus clinical isolates. The inhibition rates of bithionol biotinylated probes Bio-A2-1 and Bio-A2-3 on the biofilm formation of S. aureus were comparable to that of bithionol, and were stronger than that of Bio-A2-2 and Bio-A2-4. The biofilm formation of 10 out of 12S. aureus clinical isolates could be inhibited by Bio-A2-1 (at 1/4×, or 1/2× MICs). There are three proteins identified in S. aureus through immunoprecipitation and LC-MS/MS with bithionol biotinylated probe Bio-A2-1: Protein translocase subunit SecA 1 (secA1), Alanine--tRNA ligase (alaS) and DNA gyrase subunit A (gyrA), and in which the SecA1 protein the highest coverage and the most unique peptides. The LYS112, GLN143, ASP213, GLY496 and ASP498 of SecA1 protein act as hydrogen acceptors to form 6 hydrogen bonds with bithionol biotinylated probe Bio-A2-1 by molecular docking analysis. In conclusion, the bithionol biotinylated probe Bio-A2-1 has antibacterial and anti-biofilm activities against S. aureus, and SecA1 was probably one of the potential targets of bithionol in S. aureus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schilcher K, Horswill AR. Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol Mol Biol Rev. 2020;8:e00026–19.
Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control. 2019;8:76.
Khan F, Pham DTN, Oloketuyi SF, Kim YM. Antibiotics application strategies to control biofilm formation in pathogenic bacteria. Curr Pharm Biotechnol. 2020;21:270–86.
Ghosh A, Jayaraman N, Chatterji D. Small-molecule inhibition of bacterial biofilm. ACS Omega. 2020;5:3108–15.
Shemesh M, Kolter R, Losick R. The biocide chlorine dioxide stimulates biofilm formation in Bacillus subtilis by activation of the histidine kinase KinC. J Bacteriol. 2010;192:6352–6.
Davey ME, O’toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64:847–67.
Takeuchi T, Kobayashi S, Tanabe M, Fujiwara T. In vitro inhibition of Giardia lamblia and Trichomonas vaginalis growth by bithionol, dichlorophene, and hexachlorophene. Antimicrob Agents Chemother. 1985;27:65–70.
Amakawa M, Gunawardana S, Jabbour A, et al. Repurposing clinically approved drugs for the treatment of Bacillus cereus, a surrogate for Bacillus anthracis. ACS Omega. 2020;5:21929–39.
Leonardi W, Zilbermintz L, Cheng LW, et al. Bithionol blocks pathogenicity of bacterial toxins, ricin, and Zika virus. Sci Rep. 2016;6:34475.
Ayyagari VN, Diaz-Sylvester PL, Hsieh TJ, Brard L. Evaluation of the cytotoxicity of the Bithionol-paclitaxel combination in a panel of human ovarian cancer cell lines. PLoS One. 2017;12:e0185111.
Dayal N, Onyedibe KI, Gribble WM, Sintim HO. Membrane acting Povarov-Doebner derived compounds potently disperse preformed multidrug resistant Gram-positive bacterial biofilms. Eur J Med Chem. 2022;240:114550.
She P, Wang Y, Li Y, et al. Drug repurposing: in vitro and in vivo antimicrobial and antibiofilm effects of bithionol against Enterococcus faecalis and Enterococcus faecium. Front Microbiol. 2021;12:579806.
van Tilburg Bernardes E, Charron-Mazenod L, Reading DJ, Reckseidler-Zenteno SL, Lewenza S. Exopolysaccharide-Repressing Small Molecules with Antibiofilm and Antivirulence Activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61:e01997–16.
Kim W, Zou G, Hari TPA, et al. A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci USA. 2019;116:16529–34.
Zheng J, Shang Y, Wu Y, et al. Diclazuril inhibits biofilm formation and hemolysis of Staphylococcus aureus. ACS Infect Dis. 2021;7:1690–701.
Liu X, Xiong Y, Shi Y, et al. In vitro activities of licochalcone A against planktonic cells and biofilm of Enterococcus faecalis. Front Microbiol. 2022;13:970901.
Liu Y, Yang X, Gan J, et al. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022;50:W159–64.
Yang SP, Lin CC. Treatment of paragonimiasis with bithionol and bithionol sulfoxide. Dis Chest. 1967;52:220–32.
Barr FS, Collins GF, Wyatt LG. Potentiation of the antimicrobial activity of bithionol. J Pharm Sci. 1965;54:801–2.
Niu H, Yee R, Cui P, et al. Identification of agents active against methicillin-resistant Staphylococcus aureus USA300 from a clinical compound library. Pathogens 2017;6:44.
Lister JL, Horswill AR. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol. 2014;4:178.
Cui J, Jin J, Chaudhary AS, et al. Design, synthesis and evaluation of triazole-pyrimidine analogues as SecA inhibitors. ChemMedChem. 2016;11:43–56.
Jin J, Cui J, Chaudhary AS, et al. Evaluation of small molecule SecA inhibitors against methicillin-resistant Staphylococcus aureus. Bioorg Med Chem. 2015;23:7061–8.
Acknowledgements
The authors thank Dr. Weiguang Pan and Jie Lian (Department of Laboratory Medicine, Shenzhen Nanshan People’s Hospital and the 6th Affiliated Hospital of Shenzhen University Medical School) for helping identify and preserve the S. aureus strains.
Funding
This work was supported by the following grants: The Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture (No. XTD2210); National Natural Science Foundation of China (grants 82172283); Natural Science Foundation of Guangdong Province, China (No. 2020A1515111146, 2020A1515011049, 2020A1515010979); Shenzhen Key Medical Discipline Construction Fund (No.SZXK06162); the Science, Technology and Innovation Commission of Shenzhen Municipality basic research funds (grants JCYJ20180302144403714; JCYJ20220530141810023, JCYJ20220530142006015) and key funds JCYJ20180508162403996).
Author information
Authors and Affiliations
Contributions
YL participated in the design of this study and the synthesis of bithionol biotinylated probes Bio-A2, and drafted manuscript. ZW conducted the immunoprecipitation, participated in the measurement of the growth of S. aureus planktonic cells, and conducted LC-MS/MS experiments. YX participated in the synthesis of bithionol biotinylated probes Bio-A2, antimicrobial susceptibility testing and molecular docking. XC participated in the synthesis of bithionol biotinylated probes Bio-A2. ZS and PL collected S. aureus strains and conducted biofilm assay, and participated in the measurement of the growth of S. aureus planktonic cells. YP participated in the synthesis of bithionol biotinylated probes Bio-A2. QD and ZY participated in the design of this study, and participate in guiding and analyzing immunoprecipitation and LC-MS/MS experiments and data analysis, and reviewed this manuscript. JZ and SH designed the study, participated in the synthesis of bithionol biotinylated probes Bio-A2 and immunoprecipitation and LC-MS/MS experiments, conducted the data analysis, and provided critical revisions of the manuscript for valuable intellectual content.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures involving human subjects were approved by the institutional ethical committee of Shenzhen Nanshan People’s Hospital and the 6th Affiliated Hospital of Shenzhen University Medical School. Isolates were collected as part of the routine clinical management of patients, according to the national guidelines in China.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Y., Wen, Z., Xiong, Y. et al. The potential target of bithionol against Staphylococcus aureus: design, synthesis and application of biotinylated probes Bio-A2. J Antibiot 76, 406–415 (2023). https://doi.org/10.1038/s41429-023-00618-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00618-x