Abstract
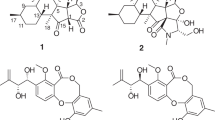
Two new antimalarial compounds, named deacetyl fusarochromene (1) and 4′-O-acetyl fusarochromanone (2), were discovered from the static fungal cultured material of Fusarium sp. FKI-9521 isolated from feces of a stick insect (Ramulus mikado) together with three known compounds fusarochromanone (3), 3′-N-acetyl fusarochromanone (4), and 5 (fusarochromene or banchromene). The structures of 1 and 2 were elucidated as new analogs of 3 by MS and NMR analyses. The absolute configurations of 1, 2, and 4 were determined by chemical derivatization. All five compounds showed moderate in vitro antimalarial activity against chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum strains, with IC50 values ranging from 0.08 to 6.35 µM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
World Health Organization, World Malaria Report 2021. https://www.who.int/publications/i/item/9789240040496
Hayashi Y, Fukasawa W, Hirose T, Iwatsuki M, Hokari R, Ishiyama A, et al. Kozupeptins, antimalarial agents produced by Paracamarosporium species: Isolation, structural elucidation, total synthesis, and bioactivity. Org Lett. 2019;21:2180–4.
Ishiyama A, Hokari R, Nonaka K, Chiba T, Miura H, Otoguro K, et al. Diatretol, an α, α’-dioxo-diketopiperazine, is a potent in vitro and in vivo antimalarial. J. Antibiot. 2021;74:266–8.
Ouchi T, Watanabe Y, Nonaka K, Muramatsu R, Noguchi C, Tozawa M, et al. Clonocoprogens A, B and C, new antimalarial coprogens from the Okinawan fungus Clonostachys compactiuscula FKR-0021. J Antibiot. 2020;73:365–71.
Watanabe Y, Hachiya K, Ikeda A, Nonaka K, Higo M, Muramatsu R, et al. Koshidacins A and B, antiplasmodial cyclic tetrapeptides from the Okinawan fungus Pochonia boninensis FKR-0564. J Nat Prod. 2022;85:2641–9.
Pathre SV, Gleason BW, Lee WY, Mirocha JC. The structure of fusarochromanone: new mycotoxin from Fusarium roseum, “Graminearum”. Can J Chem. 1986;64:1308–11.
Takahama Y, Shibata Y, Tanaka K. Concise synthesis of fungal metabolite (+)-Fusarochromanone via Rhodium (III)-catalyzed oxidative sp2 C-H bond olefination. Chem Lett. 2016;45:1177–9.
Marshall JW, Mattos-Shipley KMJ, Ghannam IAY, Munawar A, Killen JC, Lazarus CM, et al. Fusarochromene, a novel tryptophan-derived metabolite from Fusarium sacchari. Org Biomol Chem. 2021;19:182–7.
Michel DK, Ferdinand MT, Hamdi MD, Tofazzal I, Rainer BO, Hartmut L. Banchromene and other secondary metabolites from the endophytic fungus Fusarium sp. obtained from Piper guineense inhibit the motility of phytopathogenic Plasmopara viticola zoospores. Tet Lett. 2014;55:4057–61.
Kondo N, Sakai K, Kimishima A, Hokari R, Honsho M, Sato M, et al. Confluenine G, a new compound from a basidiomycetous yeast Moesziomyces sp. FKI-9540 derived from the gut of a moth Acherontia lachesis (Lepidoptera, Sphingidae). Biosci Biotechnol Biochem. 2022;86:949–54.
Nonaka K, Ōmura S, Masuma M, Kaifuchi S, Masuma R. Three new Pochonia taxa (Clavicipitaceae) from soils in Japan. Mycologia. 2013;105:1202–18.
Otoguro K, Ui H, Ishiyama A, Arai N, Kobayashi M, Takahashi Y, et al. In vitro antimalarial activities of the microbial metabolites. J Antibiot. 2003;56:322–4.
Otoguro K, Kohana A, Manabe C, Ishiyama A, Ui H, Shiomi K, et al. Potent antimalarial activities of polyether antibiotic, X-206. J Antibiot. 2001;54:658–63.
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402.
Summerell BA, Rugg CA, Burgess LW. Characterization of Fusarium babinda sp. nov. Nycol Res. 1995;99:1345–8.
Fitch RW, Snuder BB, Zhou Q, Foxman BM, Pandya AA, Yakel JL, et al. Absolute configuration and pharmacology of the poison frog alkaloid phantasmidine. J. Nat. Prod. 2018;81:1029–35.
Fraselle JV. Experimental evidence of the pathogenicity of Fusarium oxysporum Schl. F. to the oil palm (Elaeis guineensis J.). Nature. 1951;167:447.
Ying G, Xin C, Chaowei S, Karnika S, Mansoureh B, Elahe M, et al. Fusarochromanone induces G1 cell cycle arrest and apoptosis in COS7 and HEK293 cells. PloS One. 2014;11:e112641.
Matthews H, Duffy CW, Merrick CJ. Checks and balances? DNA replication and the cell cycle in Plasmodium. Parasites Vectors. 2018;11:216.
Acknowledgements
We are grateful to Distinguished Emeritus Professor Satoshi Ōmura (Kitasato University) for his helpful support and valuable guidance and suggestions. We thank Dr. Kenichiro Nagai, Ms. Reiko Seki, and Ms. Noriko Sato (School of Pharmacy, Kitasato University) for various instrumental analyses. This research was partially supported by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP21am0101096 (Phase I) and JP22ama121035 (Phase II). This work was supported by JSPS KAKENHI Grant Number JP20K07106. We would also like to thank FORTE (www.forte-science.co.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Watanabe, Y., Arakawa, E., Kondo, N. et al. New antimalarial fusarochromanone analogs produced by the fungal strain Fusarium sp. FKI-9521. J Antibiot 76, 384–391 (2023). https://doi.org/10.1038/s41429-023-00617-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00617-y
This article is cited by
-
New antimalarial iromycin analogs produced by Streptomyces sp. RBL-0292
The Journal of Antibiotics (2024)