Abstract
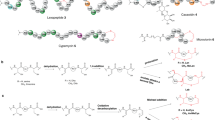
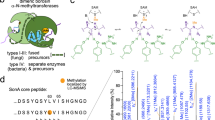
Ribosomally synthesized and posttranslationally modified peptides (RiPPs) are growing class of natural products with potent biological activities. Although the core scaffolds of RiPPs are composed of proteinogenic amino acids, remarkable structural diversity is generated through posttranslational modifications (PTMs) of precursor peptides. In addition, ribosomal origin of biosynthetic precursors enables supply of its analogs through genetic approach such as site-directed mutagenesis on corresponding genes. As PTM enzymes often exhibit substrate tolerance, RiPP biosynthetic machineries are considered as efficient tools for generation of unique peptide derivatives. RiPP pathways are distributed among all domains of life and those derived from bacteria and plants have been known for decades. In contrast, fungal RiPPs (F-RiPPs) have fewer examples. Amatoxins and omphalotins are F-RiPPs produced by Basidiomycota fungi. In the biosynthesis of these compounds, macrocyclization by prolyl oligopeptidase homologs and N-methylations of back bone amides have been characterized, respectively. Ustiloxins and related compounds are another group of F-RiPPs with characteristic macrocyclic ethers. UstYa family proteins, which are fungi-specific putative oxidases, have been identified as common proteins involved in PTMs of these compounds. Despite a limited number of characterized examples, recent progress in sequencing of fungal genomes indicated that a number of RiPP pathways are hidden in fungal resources, making F-RiPPs as attractive target for genome mining studies while more detailed understandings of key biosynthetic enzymes are still necessary. This review seeks to describe recent advances on the F-RiPP biosynthesis with slight emphasis on the function of UstYa family proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Montalbán-López M, et al. New developments in RiPP discovery, enzymology and engineering. Nat Prod Rep. 2021;38:130–239.
Arnison PG, et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30:108–60.
Kessler SC, Chooi YH. Out for a RiPP: challenges and advances in genome mining of ribosomal peptides from fungi. Nat Prod Rep. 2022;39:222–30.
Vogt E, Künzler M. Discovery of novel fungal RiPP biosynthetic pathways and their application for the development of peptide therapeutics. Appl Microbiol Biotechnol. 2019;103:5567–81.
Le Marquer M, San Clemente H, Roux C, Savelli B, Frei Dit Frey N. Identification of new signalling peptides through a genome-wide survey of 250 fungal secretomes. BMC Genomics. 2019;20:1–15.
Hallen HE, Luo H, Scott-Craig JS, Walton JD. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc Natl Acad Sci USA. 2007;104:19097–101.
Luo H, et al. Peptide macrocyclization catalyzed by a prolyl oligopeptidase involved in α-amanitin biosynthesis. Chem Biol. 2014;21:1610–7.
Luo H, et al. Genes and evolutionary fates of the amanitin biosynthesis pathway in poisonous mushrooms. Proc Natl Acad Sci USA. 2022;119:e2201113119.
Sgambelluri RM, Smith MO, Walton JD. Versatility of prolyl oligopeptidase B in peptide macrocyclization. ACS Synth Biol. 2018;7:145–52.
Czekster CM, Naismith JH. Kinetic landscape of a peptide bond-forming prolyl oligopeptidase. Biochemistry. 2017;56:2086–95.
Czekster CM, Ludewig H, McMahon SA, Naismith JH. Characterization of a dual function macrocyclase enables design and use of efficient macrocyclization substrates. Nat Commun. 2017;8:1–10.
Van Der Velden NS, et al. Autocatalytic backbone N-methylation in a family of ribosomal peptide natural products. Nat Chem Biol. 2017;13:833–5.
Ramm S, et al. A self-sacrificing N-methyltransferase is the precursor of the fungal natural product omphalotin. Angew Chem Int Ed. 2017;56:9994–7.
Song H, et al. A molecular mechanism for the enzymatic methylation of nitrogen atoms within peptide bonds. Sci Adv. 2018;4:eaat2720.
Ongpipattanakul C, Nair SK. Molecular basis for autocatalytic backbone N-methylation in RiPP natural product biosynthesis. ACS Chem Biol. 2018;13:2989–99.
Umemura M, Kuriiwa K, Viet Dao L. Tandem repeats in precursor protein stabilize transcript levels and production levels of the fungal ribosomally synthesized and post-translationally modified peptide ustiloxin B. Fungal Genet Biol. 2022;160:103691.
Yoshimi A, et al. Expression of ustR and the Golgi protease KexB are required for ustiloxin B biosynthesis in Aspergillus oryzae. AMB Express. 2016;6:1–8.
Umemura M. Peptides derived from Kex2-processed repeat proteins are widely distributed and highly diverse in the Fungi kingdom. Fungal Biol Biotechnol 2020;7:1–23.
Johnson RD, et al. A novel family of cyclic oligopeptides derived from ribosomal peptide synthesis of an in planta-induced gene, gigA, in Epichloë endophytes of grasses. Fungal Genet Biol. 2015;85:14–24.
Green KA, et al. Lolium perenne apoplast metabolomics for identification of novel metabolites produced by the symbiotic fungus Epichloë festucae. N. Phytol. 2020;227:559–71.
Nagano N, et al. Class of cyclic ribosomal peptide synthetic genes in filamentous fungi. Fungal Genet Biol. 2016;86:58–70.
Umemura M, et al. MIDDAS-M: Motif-independent de novo detection of secondary metabolite gene clusters through the integration of genome sequencing and transcriptome data. PLoS One. 2013;8:e84028.
Umemura M, et al. Characterization of the biosynthetic gene cluster for the ribosomally synthesized cyclic peptide ustiloxin B in Aspergillus flavus. Fungal Genet Biol. 2014;68:23–30.
Ye Y, et al. Unveiling the biosynthetic pathway of the ribosomally synthesized and post-translationally modified peptide ustiloxin B in filamentous fungi. Angew Chem Int Ed 2016;55:8072–5.
Ellis JM, et al. Biocatalytic synthesis of non-standard amino acids by a decarboxylative aldol reaction. Nat Catal. 2022;5:136–43.
Ding W, et al. Biosynthetic investigation of phomopsins reveals a widespread pathway for ribosomal natural products in Ascomycetes. Proc Natl Acad Sci USA. 2016;113:3521–6.
Sogahata K, et al. Biosynthetic studies of phomopsins unveil posttranslational installation of dehydroamino acids by UstYa family proteins. Angew Chem Int Ed 2021;60:25729–34.
Kessler SC, et al. Victorin, the host-selective cyclic peptide toxin from the oat pathogen Cochliobolus victoriae, is ribosomally encoded. Proc Natl Acad Sci. 2020;117:24243–50.
Ye Y, et al. Heterologous production of asperipin-2a: Proposal for sequential oxidative macrocyclization by a fungi-specific DUF3328 oxidase. Org Biomol Chem 2019;17:39–43.
Shabani S, White JM, Hutton CA. Total synthesis of the putative structure of asperipin-2a and stereochemical reassignment. Org Lett. 2020;22:7730–4.
Jiang Y, et al. Biosynthesis of cyclochlorotine: identification of the genes involved in oxidative transformations and intramolecular O,N-Transacylation. Org Lett 2021;23:2616–20.
Schafhauser T, et al. The cyclochlorotine mycotoxin is produced by the nonribosomal peptide synthetase CctN in Talaromyces islandicus (‘ Penicillium islandicum’). Environ Microbiol. 2016;18:3728–41.
Schafhauser T, et al. Antitumor astins originate from the fungal endophyte Cyanodermella asteris living within the medicinal plant Aster tataricus. Proc Natl Acad Sci USA. 2019;116:26909–17.
Gadsby DC, Vergani P, Csanády L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–83.
Kim H, Nelson MA. Molecular and functional analyses of poi-2, a novel gene highly expressed in sexual and perithecial tissues of Neurospora crassa. Eukaryot Cell. 2005;4:900–10.
Kersten RD, Weng JK. Gene-guided discovery and engineering of branched cyclic peptides in plants. Proc Natl Acad Sci USA. 2018;115:E10961–E10969.
Chigumba DN, et al. Discovery and biosynthesis of cyclic plant peptides via autocatalytic cyclases. Nat Chem Biol. 2022;18:18–28.
Macko V, et al. Characterization of victorin C, the major host-selective toxin from Cochliobolus victoriae: Structure of degradation products. Experientia. 1985;41:1366–70.
Funding
This work was financially supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (JSPS KAKENHI Grant Number JP19H02891 (HO), JP22H02204 (AM), JP16H06446 (AM) and JP19H04635 (TO) and Institute for Fermentation, Osaka (IFO, Grant Number G-2022-3-011) (AM).
Author information
Authors and Affiliations
Contributions
TO, AM, and HO wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozaki, T., Minami, A. & Oikawa, H. Recent advances in the biosynthesis of ribosomally synthesized and posttranslationally modified peptides of fungal origin. J Antibiot 76, 3–13 (2023). https://doi.org/10.1038/s41429-022-00576-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-022-00576-w