Abstract
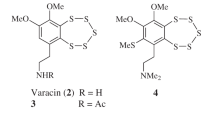
Dialkylresorcinols are a class of antimicrobial natural products produced by a range of bacterial species. Semi-synthetic derivatization of two microbial dialkylresorcinols isolated from a Pseudomonas aurantiaca strain has yielded 21 derivatives, which were tested for antimicrobial activity, revealing several trends in their activity. The presence of aromatic and phenolic hydrogen atoms was crucial for activity, with all derivatives lacking these features possessing greatly reduced activity. On the other hand, derivatives with shorter alkyl chains at C-5 possessed lower MIC values, while one mono-fluorosulfated derivative showed significantly improved activity against several of the test strains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kanda N, Ishizaki N, Inoue N, Oshima M, Handa A, Kitahara T. DB-2073, a new alkylresorcinol antibiotic-I. Taxonomy, isolation and characterization. J Antibiot. 1975;28:935–42.
Schöner TA, Kresovic D, Bode HB. Biosynthesis and function of bacterial dialkylresorcinol compounds. Appl Microbiol Biotechnol. 2015;99:8323–8.
Sankawa U, Shimada H, Yamasaki K. Biosynthesis of 2-hexyl-5-propylresorcinol: biosynthetic incorporation of deuterium from [2-13C, 2-2H3]-acetate. Chem Pharm Bull. 1981;29:3601–5.
Nowak-Thompson B, et al. 2,5-Dialkylresorcinol biosynthesis in Pseudomonas aurantiaca: novel head-to-head condensation of two fatty acid-derived precursors. J Bacteriol. 2003;185:860–9.
Fuchs SW, et al. Formation of 1,3-cyclohexanediones and resorcinols catalyzed by a widely occuring ketosynthase. Angew Chem. 2013;52:4108–12.
Kitahara T, Kanda N. DB-2073, a new alkylresorcinol antibiotic-II. The chemical structure of DB-2073. J Antibiot. 1975;28:943–6.
Anton P, Jolanta L, Anders B. Antimicrobial dialkylresorcinols from Pseudomonas sp. Ki19. J Nat Prod. 2006;69:654–7.
Arisawa M, Ohmura K, Kobayashi A, Morita N. A cytotoxic constituent of Lysimachia japonica THUNB. (Primulaceae) and the structure-activity relationships of related compounds. Chem Pharm Bull 1989;37:2431–4.
Kato S, Shindo K, Kawai H, Matsuoka M, Mochizuki J. Studies on free radical scavenging substances from microorganisms. J Antibiot 1993;46:1024–6.
Sophie B, Darko K, Helge BB, Ralf H. Dialkylresorcinols as bacterial signaling molecules. Proc Natl Acad Sci USA. 2015;112:572–7.
Sophie B, Helge BB, Ralf H. Languages and dialects: bacterial communication beyond homoserine lactones. Trends Microbiol 2015;23:521–3.
Covarrubias-Zúñiga A, Avila-Zárraga JG, Arias-Salas D. A total synthesis of the antibiotic DB-2073. Synth Commun 2003;33:3173–81.
Sébastien C, Jean-Pierre F. A simple synthesis of the natural 2,5-dialkylresorcinol free radical scavenger antioxidant: resorstatin. Synth Commun 1997;27:3769–78.
Madhavachary R, Ramachary DB. High-yielding total synthesis of sexually deceptive chiloglottones and antimicrobial dialkylresorcinols through an organocatalytic reductive coupling reaction. Eur J Org Chem. 2014;2014:7317–23.
Yoneyama H, Katsumata R. Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci Biotech Bioch. 2006;70:1060–75.
Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–61.
Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670–95.
Barnes EC, Kumara R, Davis RA. The use of isolated natural products as scaffolds for the generation of chemically diverse screening libraries for drug discovery. Nat Prod Rep. 2016;33:372–81.
Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–76.
Ren YL, Kinghorn AD. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019;85:802–14.
Ville A, Viault G, Hélesbeux JJ, Richomme DGP, Séraphin D. Efficient semi-synthesis of natural-(R)-tocotrienols from a renewable vegetal source. J Nat Prod. 2019;82:51–8.
Deng Y, Tang D, Wang Q-R, Huang S, Fu L-Z. Semi-synthesis, antibacterial activity, and molecular docking study of novel pleuromutilin derivatives bearing cinnamic acids moieties. Arch Pharm Chem Life Sci. 2019;352:e1800266.
Mittal N, Tesfu HH, Hogan AM, Cardona ST, Sorensen JL. Synthesis and antibiotic activity of novel acylated phloroglucinol compounds against methicillin-resistant Staphylococcus aureus. J Antibiot 2019;72:253–9.
Caprioglio D, et al. O-Methyl phytocannabinoids: Semi-synthesis, analysis in cannabis flowerheads, and biological activity. Planta Med 2019;85:981–6.
Shi Y, Zaleta Pinet DA, Clark BR. Isolation, identification, and composition of antibacterial dialkylresorcinols from a Chinese Pseudomonas aurantiaca strain. J Nat Prod. 2020;83:194–201.
Budzikiewicz H, Scholl H, Neuenhaus W, Pulverer G, Korth H. Dialkyl resorcinols from Pseudomonas aureofaciens. Z Naturforsch B. 1980;35B:909–10.
Liu Z, et al. SuFEx click chemistry enabled late-stage drug functionalization. J Am Chem Soc. 2018;140:2919–25.
Cheng AV, Wuest WM. Signed, sealed, delivered: conjugate and prodrug strategies as targeted delivery vectors for antibiotics. ACS Infect Dis. 2019;5:816–28.
Rahman M, Kuhn I, Rahman M, Olsson-Liljequist B, Mollby R. Evaluation of a scanner-assisted colorimetric MIC method for susceptibility testing of Gram-negative fermentative bacteria. Appl Environ Microbiol. 2004;70:2398–403.
Li K, Lin G, Zhu J, Wu H, Sun Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan-gallic acid derivative against Escherichia coli and Staphylococcus aureus. Food Control 2019;96:234–43.
Acknowledgements
This work was funded in part by the award from the Research Fund for International Young Scientists (81850410550) from the National Science Foundation of China, and by a grant from the National Basic Research Program of China (2015CB856500). The authors would like to acknowledge Dr. R. Srinivasan for his helpful comments during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, J., Shi, Y. & Clark, B.R. Semi-synthesis of antibacterial dialkylresorcinol derivatives. J Antibiot 74, 70–75 (2021). https://doi.org/10.1038/s41429-020-0359-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-0359-5