Abstract
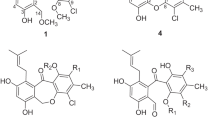
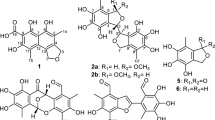
Six new butyrolactone derivatives (1, 2a/2b, 3a/3b and 4), together with another two known derivatives (5 and 6) were isolated from the endophytic fungus Talaromyces sp. CPCC 400783. Their structures were established by a combination of spectroscopic analysis, including NMR and HRESIMS. The absolute configurations were elucidated by ECD experiments. Subsequently, compound 1, 3b, 4 and 5 exhibited good inhibitory effect against influenza A/WSN/33 (H1N1) virus with IC50 values of 21.93 ± 1.51, 21.54 ± 3.75, 18.36 ± 2.15 and 23.80 ± 3.05 μM respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ancheeva E, Daletos G, Proksch P. Bioactive secondary metabolites from endophytic fungi. Curr Med Chem. 2020;27:1836–54.
Kharwar RN, Mishra A, Gond SK, Stierle A, Stierle D. Anticancer compounds derived from fungal endophytes: their importance and future challenges. Nat Prod Rep. 2011;28:1208–28.
Nisa H, Kamilia AN, Nawchoo IA, Shafia S, Shameema N, Bandha SA. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb Pathog. 2015;82:50–59.
Liu JM, Zhang DW, Zhang M, Chen RD, Yan Z, Zhao JY, et al. Periconones B-E, new meroterpenoids from endophytic fungus Periconia sp. Chinese Chem Lett. 2017;2:248–52.
Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol. 2014;78:175–341.
He JW, Liang HX, Gao H, Kuang RQ, Chen GD, Hu D, et al. Talaflavuterpenoid A, a new nardosinane-type sesquiterpene from Talaromyces flavus. J Asian Nat Prod Res. 2014;16:1029–34.
He JW, Mu ZQ, Gao H, Chen GD, Zhao Q, Dan HU, et al. New polyesters from Talaromyces flavus. Tetrahedron. 2014;70:4425–30.
Xie XS, Fang XW, Huang R, Zhang SP, Wei HX, Wu SH, et al. A new dimeric anthraquinone from endophytic Talaromyces sp. YE3016. Nat Prod Res. 2016;30:1706–11.
Bara R, Aly AH, Pretsch A, Wray V, Wang BG, Proksch P, et al. Antibiotically active metabolites from Talaromyces wortmannii, an endophyte of Aloe vera. J Antibiot. 2013;66:491–93.
Chu YS, Niu XM, Wang YL, Guo JP, Pan WZ, Huang XW, et al. Isolation of putative biosynthetic intermediates of prenylated indole alkaloids from a thermophilic fungus Talaromyces thermophilus. Org Lett. 2010;12:4356–59.
Guo JP, Tan JL, Wang YL, Wu HY, Zhang CP, Niu XM, et al. Isolation of talathermophilins from the thermophilic fungus Talaromyces thermophilus YM3-4. J Nat Prod. 2011;74:2278–81.
Frisvad JC. Taxonomy, chemodiversity, and chemoconsistency of Aspergillus, Penicillium, and Talaromyces species. Front Microbiol. 2015;5:773–79.
Liu J, Peng C, He CJ, Liu JL, He YC, Guo L, et al. New amino butenolides from the bulbs of Fritillaria unibracteata. Fitoterapia. 2014;98:53–58.
Bai ZQ, Lin XP, Wang YZ, Wang JF, Zhou XF, Yang B, et al. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia. 2014;95:194–202.
Liu HT, Liu SC, Guo LD, Zhang YG, Cui LJ, Ding G. New furanones from the plant endophytic fungus Pestalotiopsis besseyi. Molecules. 2012;17:14015–21.
Ma XH, Zhu TJ, Gu QQ, Xi R, Wang W, Li DH. Structures and antiviral activities of butyrolactone derivatives isolated from Aspergillus terreus MXH-23. J Ocean U China. 2014;13:1067–70.
Wang WH, Kim HY, Patil RS, Giri AG, Wang HD, Hahn DY, et al. Cadiolides J-M, antibacterial polyphenyl butenolides from the Korean tunicate Pseudodistoma antinboja. Bioorg Med Chem Lett. 2017;27:574–7.
Wang W, Kim H, Nam SJ, Rho BJ, Kang H. Antibacterial butenolides from the Korean tunicate Pseudodistoma antinboja. J Nat Prod. 2012;75:2049–54.
Wang R, Liu TM, Shen MH, Yang MQ, Feng QY, Tang XM, et al. Spiculisporic acids B-D, three new γ-butenolide derivatives from a sea urchin-derived fungus Aspergillus sp. HDf2. Molecules. 2012;17:13175–82.
Guo F, Li ZL, Xu XW, Wang KB, Shao ML, Feng Z, et al. Butenolide derivatives from the plant endophytic fungus Aspergillus terreus. Fitoterapia. 2016;113:44–50.
An X, Pei YH, Chen SF, Li SG, Hu XL, Chen G, et al. Three New butenolides from the fungus Aspergillus sp. CBS-P-2. Molecules. 2016;21:1361–71.
Zhang LH, Feng BM, Zhao YQ, Sun Y, Liu B, Liu F, et al. Polyketide butenolide, diphenyl ether, and benzophenone derivatives from the fungus Aspergillus flavipes PJ03-11. Bioorg Med Chem Lett. 2016;26:346–50.
Gao Q, Wang Z, Liu ZL, Li XY, Zhang YX, Zhang ZZ, et al. A cell-based high-throughput approach to identify inhibitors of influenza A virus. Acta Pharm Sin B. 2014;4:301–06.
Yang YN, Huang XY, Feng AM. New butyrolactone type lignans from Arctii Fructus and their anti-inflammatory activities. J Agric Food Chem. 2015;63:7958–66.
Raffauf RF, Zennie TM, Onan KD, Quesne PW. Funebrine, a structurally novel pyrrole alkaloid, and other γ-hydroxyisoleucine-related metabolites of Quararibea funebris (llave) vischer (bombacaceae). J Org Chem. 1984;49:2714–18.
Nonaka K, Chiba T, Suga T, Asami Y, Iwatsuki M, Masuma R, et al. Coculnol, a new penicillic acid produced by a coculture of Fusarium solani FKI-6853 and Talaromyces sp. FKA-65. J Antibiot. 2015;68:530–32.
Qian PY, Wong YH, Zhang Y. Changes in the proteome and phosphoproteome expression in the bryozoan Bugula neritina larvae in response to the antifouling agent butenolide. Proteomics. 2010;10:3435–46.
Phainuphong P, Rukachaisirikul V, Tadpetch K, Sukpondma Y, Saithong S, Phongpaichit S, et al. γ-Butenolide and furanone derivatives from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. Phytochemistry. 2017;137:165–73.
Burkardt HJ. Pandemic H1N1 2009 ('swine flu'): diagnostic and other challenges. Expert Rev Mol Diagn. 2011;11:35–40.
Zhang J, Liu T, Tong XM, Li G, Yan JH, Ye X. Identification of novel virus inhibitors by influenza A virus specific reporter cell based screening. Antiviral Res. 2012;93:48–54.
Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–26.
Acknowledgements
This work was financially supported by the Drug Innovation Major Project (2018ZX09711001-007-001), the National Natural Science Foundation of China (No. 81973220), CA and MS Innovation Fund for Medical Sciences (No. 2016-I2M-2-002, 2016-I2M-2-003 and 2020-I2M-2-010), the National Microbial Resource Center (No. NMRC-2020-3) and Practical training plan for cross training of high level talents in Beijing Universities (No. 2018-160).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, R., He, W., Wang, Y. et al. New butyrolactone derivatives from the endophytic Fungus Talaromyces sp. CPCC 400783 of Reynoutria japonica Houtt. J Antibiot 74, 225–232 (2021). https://doi.org/10.1038/s41429-020-00388-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-00388-w