Abstract
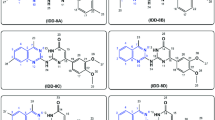
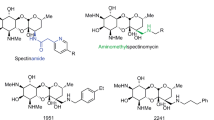
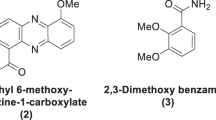
To find novel polymycin analogues with high antimicrobial activities and low toxicity, 36 novel polymyxin analogues were synthesized, and in which TZ40-J and TZ40-K were evaluated for their antimicrobial activities using broth microdilution method and for their haemolytic toxicity with sterile sheep blood. Preliminary safety assessments of those two compounds were carried out via the MTT cell viability assay in vitro and acute toxicity assay in vivo. Experimental data demonstrated that TZ40-J and TZ40-K were less toxic and indicate higher activities against Pseudomonas aeruginosa than polymyxin B.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jiang X, et al. Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat Commun. 2017;8:15784.
Vaara M. Novel derivatives of polymyxins. J Antimicrob Chemother. 2013;68:1213–19.
Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145.
Vaara M, Vaara T, Tyrrell JM. Structure–activity studies on polymyxin derivatives carrying three positive charges only reveal a new class of compounds with strong antibacterial activity. Peptides. 2017;91:8–12.
Calonge N, et al. Screening for asymptomatic bacteriuria in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2008;149:43–47.
Velkov T, Thompson PE, Nation RL, Li J. Structure—activity relationships of polymyxin antibiotics. J Med Chem. 2010;53:1898–916.
Falagas ME, Rafailidis PI. Nephrotoxicity of colistin: new insight into an old antibiotic. Clin Infect Dis. 2009;48:1729–31.
Antoniadou A, et al. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J Antimicrob Chemother. 2007;59:786–90.
Li J, Rayner CR, Nation RL, Owen RJ, Tan KE, Spelman D. Hetero-resistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946–50.
Garonzik SM, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284–94.
Roberts KD, Li J, Velkov T, Nation RL, Thompson PE. Antimicrobial polymyxin derivative compounds. AU; WO: 2017054047A1. 2017.
Vaara M, Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983;303:526–8.
Nikaido H. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32.
Hancock RE. Antibacterial peptides and the outer membranes of Gram negative bacilli. J Med Microbiol. 1997;46:1–3.
Hancock RE, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–8.
Thomas CJ, Surolia A. Kinetics of the interaction of endotoxin with polymyxin B and its analogs: a surface plasmon resonance analysis. FEBS Lett. 1999;445:420–4.
Thomas CJ, Surolia N, Surolia A. Surface plasmon resonance studies resolve the enigmatic endotoxin neutralizing activity of polymyxin B. J Biol Chem. 1999;274:29624–7.
Bergen PJ, et al. Pharmacokinetics and pharmacodynamics of ‘old’ polymyxins: what is new? Diagnostic Microbiol Infect Dis. 2012;74:213–23.
Zhang Y, Tang HQ, Wang Y, Zhao WJ, Dong YZDesign. Synthesis and antibacterial activity of a group of polymyxin analogues. Chin J Pharm. 2018;10:1378–87.
Tsubery H, Ofek I, Cohen S, Fridkin M. N-terminal modifications of polymyxin nonapeptide and their effect on antibacterial activity. Peptides. 2001;22:1675–81.
Katsuma N, et al. Development of desfatty acyl-polymyxin B decapeptide analogs with Pseudomonas aeruginosa-specific antimicrobial activity. Chem Pharm Bull. 2009;57:332–6.
Doymaz MZ, Karaaslan E. Comparison of antibacterial activities of polymyxin B and colistin against multidrug resistant Gram negative bacteria. Infect Dis. 2019. https://doi.org/10.1080/23744235.2019.1640386.
Tsubery H. Modulation of the hydrophobic domain of polymyxin B nonapeptide: effect on outer-membrane permeabilization and lipopolysaccharide neutralization. Mol Pharmacol. 2002;62:1036–42.
Ling LL, et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–9.
CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard-second edition, CLSI documentM-27A2. vol. 22. Wayne, PA: Clinical and Laboratory Standards Institute; 2002.
Bulmus V, Woodward M, Lin L, Murthy N, Stayton P, Hoffman A. A new pH-responsive and glutathione- reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J Control Release. 2003;93:105–20.
Acknowledgements
We thank Chitai Tianqing Pharmaceutical Group Co. LTD for acute toxicity assay support.
Funding
This work was supported by the Ministry of Science and Technology of the People’s Republic of China, the national key research and development program (program reference: 2017YFD0501404).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tang, H., Zhang, Y., Ma, J. et al. Design, synthesis and antimicrobial studies of some polymyxin analogues. J Antibiot 73, 158–166 (2020). https://doi.org/10.1038/s41429-019-0262-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0262-0
This article is cited by
-
A pH/enzyme dual responsive PMB spatiotemporal release hydrogel promoting chronic wound repair
Journal of Nanobiotechnology (2023)
-
Combination of Derivatization–HPLC–MS and Enzymatic Hydrolysis–Edman Degradation for Amino Acid Sequence and Configuration of Polymyxin B Components
Chromatographia (2021)