Abstract
Endoplasmic reticulum (ER) stress and the subsequent adaptive cellular response, termed the unfolded protein response (UPR), have been implicated in several diseases, including cancer. In this review, I present a brief introduction to ER stress and the UPR and then summarize the importance of the IRE1α-XBP1 branch as a target for anticancer drug discovery. In addition, I introduce our approach to the identification of inhibitors against the IRE1α-XBP1 branch from microbial cultures. As a result of our screening, toyocamycin has been identified and toyocamycin showed anticancer activity against multiple myeloma.
Similar content being viewed by others
Introduction
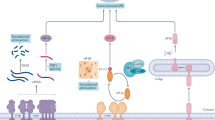
The endoplasmic reticulum (ER) is an organelle that plays an important role in several processes, including Ca2+ homeostasis, folding of newly synthesized proteins, and post-translational modification of proteins. Only properly folded and modified proteins can exit the ER. However, deprivation of essential nutrients, the presence of mutations, and several other types of stimuli in the synthesized proteins themselves, alone or in combination, have been shown to perturb the function of the ER. This perturbation results in the accumulation of unfolded proteins in the ER, which are harmful to cells and lead to so-called ER stress. Therefore, in order to maintain homeostasis in the ER, the cells activate an adaptation system known as the unfolded protein response (UPR). The UPR consists of the following four cellular responses: (1) pausing of protein translation, in order to limit the load of new proteins in the ER, through inactivation of eukaryotic initiation factor 2 (eIF2); (2) upregulation of molecular chaperones and folding enzymes, such as glucose-regulated protein 78 (GRP78, also known as BiP), GRP94, protein disulfide isomerase (PDI), and ER-localized DnaJ 4 (ERdj4), to enhance the capacity of the protein folding system; (3) activation of the ER-associated degradation system (ERAD) in order to eliminate the unfolded proteins from the ER; (4) activation of apoptosis machinery in the event of failure to eliminate the unfolded proteins from the ER. The UPR is initiated by the activation of three ER membrane-bound proteins that sense the presence of unfolded proteins in the ER. These ER stress sensors are inositol-requiring enzyme 1α (IRE1α), protein kinase regulated by RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (Fig. 1a).
Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR). a Schematic illustration of ER stress and the UPR. ER stress is induced by several stimuli, including ER stress-inducing compounds such as tunicamycin and thapsigargin, microenvironmental changes such as nutrient deprivation and low pH, and gene mutation. ER stress activates three ER stress sensors (IRE1α, ATF6, and PERK) to induce the UPR. The UPR is governed by the upregulation of UPR target genes that are involved in the ER-associated degradation system (ERAD), protein folding, and the induction of apoptosis. b Schematic illustration of IRE1α-mediated cleavage of XBP1 mRNA
The three ER stress sensors
Of the three ER stress sensors, IRE1α is the most evolutionarily conserved and was the first to be discovered as an ER stress sensor. IRE1α encodes an ER-localized type I transmembrane protein with a luminal domain in the N-terminal region and protein serine/threonine kinase and RNase domains in the C-terminal region [1, 2]. It has been shown that dimerization and oligomerization of IRE1α leads to trans-autophosphorylation, resulting in activation of the RNase domain [3, 4]. The exact mechanism of IRE1α activation in response to the accumulation of unfolded proteins is not entirely clear. However, structural and biophysical studies have proposed a model of unfolded protein-induced IRE1α activation and subsequent activation of XBP1, a transcription factor containing bZIP (basic leucine zipper) domain, as follows (Fig. 1b): (1) GRP78 is known to maintain IRE1α in an inactive state by binding to the luminal domain of IRE1α. However, upon accumulation of unfolded proteins in the ER, GRP78 dissociates from IRE1α in order to bind to unfolded proteins. This dissociation of GRP78 from IRE1α allows the luminal domain of IRE1α to undergo dimerization [5]; (2) dimerization or oligomerization of IRE1α occurs; (3) the dimerized/oligomerized IRE1α leads to trans-autophosphorylation of the kinase activation loop domains, which results in a conformational change [3]; (4) this conformational change permits cofactor (ADP or ATP) binding, promoting back-to-back dimer configuration of the cytosolic domains [4, 6]; (5) oligomerization of the cytosolic domain activates the RNase activity of IRE1α, which subsequently cleaves XBP1 mRNA at two sites to initiate an unconventional splicing reaction [4]. IRE1α-induced cleavage of XBP1 mRNA results in the removal of a 26-nucleotide intron and the 5′ and 3′ fragments are subsequently joined by RNA ligase activity. This unconventional splicing reaction creates a translational frameshift to produce the active XBP1 transcription factor [7, 8]. The activated XBP1 transactivates a subset of target genes that are involved in protein folding, ERAD, protein translocation to the ER, and protein secretion.
PERK is also an ER-localized type I transmembrane serine/threonine protein kinase. Under normal conditions, PERK is held in an inactive state through the association of its luminal domain with GRP78. However, accumulation of excess unfolded proteins in the ER results in the dissociation of GRP78 from PERK, which in turn causes dimerization/oligomerization of PERK [5]. The dimerized/oligomerized PERK induces trans-autophosphorylation, and the phosphorylated PERK phosphorylates the α subunit of eukaryotic translation initiation factor 2 (eIF2α) [9, 10]. Phosphorylated eIF2α is known to indirectly inactivate eIF2, resulting in inhibition of mRNA translation (Fig. 1a).
ATF6, a transcription factor, is an ER-localized type-II transmembrane protein in which the N-terminal cytoplasmic region contains a bZIP and DNA transactivation domain and the C-terminal luminal region senses unfolded proteins. Upon accumulation of unfolded proteins in the ER, ATF6 is translocated from the ER to the Golgi apparatus [11]. In the Golgi apparatus, ATF6 is cleaved by two proteases, S1P and S2P (site-1 and site-2 protease), which results in the production of ATF6 N-terminal cytosolic fragment (ATF6[N]) [12, 13]. ATF6(N) then moves into the nucleus and acts as a transcription factor to regulate UPR target genes (Fig. 1a).
These three ER stress sensors cooperate to control the expression of UPR target genes.
UPR and diseases
ER stress and the UPR have been reported to contribute to several diseases and conditions, including cancer, neurodegenerative disorders, diabetes, and inflammation.
It is well known that tumor cells can grow under a variety of stressful conditions, such as hypoxia, nutrient deprivation, low pH, or poor vascularization. In these stressful conditions, unfolded proteins are known to accumulate in the ER of tumor cells, resulting in UPR induction. Tumor cells activate the UPR in order to alleviate these stresses and restore ER homeostasis, promoting cell survival and adaptation. Indeed, several studies have reported involvement of the UPR in cancer development. High levels of GRP78 expression have been observed in breast [14], prostate [15], colorectal [16], and ovarian cancers [17], as well as glioma [18]. The expression of XBP1 has also been reported to increase in several types of tumor cells, such as breast cancer [19], hepatocellular carcinoma [20], and multiple myeloma (MM) cells [21]. Moreover, XBP1-deficient cells have been reported to be sensitive to hypoxia-induced apoptosis, and loss of XBP1 inhibited tumor growth in vivo [22]. The activity of XBP1 has been shown to be strongly correlated with the expression of HIF1α in triple-negative breast cancer [23]. Thus, it has been suggested that XBP1 is an essential survival factor for tumor growth. On the other hand, PERK levels have also been reported to be correlated with tumor growth. Nrf2, a transcription factor that regulates cellular redox homeostasis, is a direct substrate of PERK [24]. In PERK-knockdown cells, the activity of Nrf2 was reduced, leading to the induction of oxidative DNA damage. Therefore, tumor volumes were reduced in a PERK-deficient mouse mammary tumor model [25]. Moreover, the PERK inhibitor GSK2656157 showed anti-tumor activity in human tumor xenograft models of pancreatic cancer cells [26]. These studies suggest the importance of the UPR, and especially XBP1, in cancer development.
The UPR also acts as a cytoprotectant in neurodegenerative disorders. In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of Parkinson’s disease, ATF6 knockout increased the loss of dopaminergic neurons [27], while the enforced the expression of an active form of XBP1 (XBP1s) by using an adenovirus suppressed the degeneration of dopaminergic neurons [28]. Furthermore, the enforced expression of XBP1s in the striatum by adenoviral transduction reduced aggregation of mutant Huntingtin in a mouse model of Huntington’s disease [29].
ER stress is reported to be a central feature of insulin resistance and diabetes. In the liver tissue of both high-fat diet and ob/ob mice, which are well-known diabetes models, expression of GRP78 and phosphorylation levels of PERK and eIF2α were elevated. Moreover, mice deficient in XBP1 developed insulin resistance [30]. In addition, chemical chaperons, such as 4-phenyl butyric acid (PBA) [31] and taurine-conjugated ursodeoxycholic acid (TUDCA) [32], are known to stabilize protein conformation, leading to improved ER folding capacity. These compounds reversed insulin resistance in the liver tissue of ob/ob mice [33]. These results suggest that ER stress is involved in the development of diabetes and insulin resistance. On the other hand, overexpression of XBP1s has been reported to improve insulin resistance in ob/ob mice. The PI3K-Akt pathway is activated upon insulin stimulation, leading to phosphorylation of FOXO1, which is a transcription factor that regulates gluconeogenesis. The phosphorylated form of FOXO1 by Akt is known to be localized in the cytoplasm through 14-3-3 binding, which results in inactivation of FOXO1 and inhibition of gluconeogenesis. XBP1s interact with FOXO1, resulting in proteasome-mediated degradation of FOXO1 and decreasing hepatic gluconeogenesis [34]. Therefore, XBP1 is considered to be a therapeutic target for diabetes.
Inhibitors against IRE1α-mediated activation of XBP1
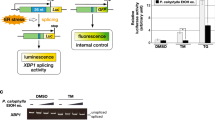
As mentioned above, the IRE1α-XBP1 branch is considered to be a therapeutic target for malignant tumors. Therefore, we tried to identify inhibitors against ER stress-induced activation of the IRE1α-XBP1 branch. To accomplish this, we implemented a novel screening system based on the mechanism of IRE1α-mediated XBP1 activation in order to easily detect activity of the IRE1α-XBP1 branch. First, we constructed a pcDNA3/XBP1-luciferase plasmid, in which human XBP1 cDNA was fused upstream of luciferase cDNA (designated as XBP1-Luc). When this construct is transfected into mammalian cells, the luciferase protein is not expressed because translation is terminated at a stop codon located upstream of luciferase mRNA. Under ER stress conditions, however, ER stress-mediated splicing of a 26-base XBP1 mRNA causes a frameshift in XBP1-Luc mRNA. Therefore, the translation from XBP1-Luc mRNA is terminated at the stop codon of luciferase mRNA, resulting in the expression of full-length XBP1-Luc protein. Next, we established a line of HeLa cells in which XBP1-Luc was stably expressed (Fig. 2a).
Screening to identify inhibitors of the IRE1α-XBP1 branch. a Schematic illustration of the screening system we developed. Under normal condition, the luciferase protein are not be expressed because translation is terminated at a stop codon located upstream of luciferase mRNA. However, under ER stress condition, a 26-base XBP1 mRNA is removed by ER stress-mediated splicing, which resulted in a frameshift in XBP1-Luc mRNA. XBP1-luciferase fusion protein can be expressed. Therefore, we can easily evaluate the activity of IRE1α-XBP1 branch by luciferase reporter assay. b Structures of trierixin and quinotrierixin that were identified as inhibitors against ER stress-induced activation of XBP1. c Effects of nucleotide analogs against ER stress-induced activation of XBP1
Trierixin and quinotrierixin
Based on our assay system, we screened inhibitors against ER stress-induced activation of the IRE1α-XBP1 branch from the culture broth of microorganism. As a result, we obtained two novel triene-ansamycin group compounds, namely trierixin [35, 36] and quinotrierixin [37, 38], from Streptomyces strains (Fig. 2b). Quinotrierixin-producing strains produced several triene-ansamycin group compounds; therefore, we isolated some of these and performed a structure–activity relationship (SAR) study. Our SAR study of 12 triene-ansamycin group compounds showed that their inhibitory activities were correlated with XBP1 activation and tumor cell growth. However, trierixin and quinotrierixin suppressed ER stress-induced activation of not only the IRE1α-XBP1 branch, but also the PERK and ATF6 branches.
In addition, another triene-ansamycin group compound, cytotrienin A [39], has been reported to inhibit protein synthesis [40], suggesting that trierixin and quinotrierixin may also be protein synthesis inhibitors. Indeed, they did inhibit protein synthesis, as evaluated based on [3H]-leucine incorporation into the macromolecular fraction, at the same concentration that inhibited ER stress-induced activation of XBP1. It has therefore been suggested that they suppress the accumulation of unfolded proteins in the ER through inhibition of protein synthesis, leading to inhibition of XBP1 activation [41].
Toyocamycin
We performed further screening and obtained toyocamycin from a culture broth of a strain of Actinomycete [42] (Fig. 2b). Toyocamycin was originally identified as an anti-candida antibiotic [43], and was later reported to inhibit RNA synthesis [44]. Given that protein-synthesis inhibitors suppressed ER stress-induced XBP1 activation [41], we speculated that toyocamycin may also inhibit ER stress-induced XBP1 activation by suppressing the accumulation of unfolded proteins through inhibition of RNA synthesis. However, the IC50 value of toyocamycin as an inhibitor of XBP1 activation was 100-fold less than that as an inhibitor of RNA synthesis. Moreover, a well-known RNA synthesis inhibitor, actinomycin D, did not inhibit ER stress-induced activation of XBP1. Therefore, the inhibitory activity of toyocamycin against ER stress-induced XBP1 activation was not due to inhibition of RNA synthesis.
As mentioned above, the accumulation of unfolded proteins in the ER induces dimerization/oligomerization of IRE1α, which results in trans-autophosphorylation of IRE1α and subsequent cleavage of XBP1 mRNA. Toyocamycin has been reported to inhibit the activity of protein kinases such as PKC [45], cdc2 [46], and PI4K [47]. Therefore, we wondered whether toyocamycin inhibits phosphorylation of IRE1α. Overexpression of IRE1α has been reported to induce homo-oligomerization and subsequent autophosphorylation at Ser724 [48, 49]. Thus, we examined the effect of toyocamycin on IRE1α phosphorylation at Ser724 in IRE1α-overexpressing 293T cells. While toyocamycin inhibited XBP1 mRNA splicing induced by overexpression of IRE1α, it did not inhibit IRE1α phosphorylation at Ser724. This suggests that toyocamycin does not inhibit the autophosphorylation of IRE1α. On the other hand, it did inhibit IRE1α-mediated XBP1 mRNA cleavage in vitro, indicating that it inhibits the RNase activity of IRE1α, which is regulated by autophosphorylation and subsequent cofactor (ADP or ATP) binding to IRE1α. Therefore, it seems that toyocamycin inhibits cofactor binding to IRE1α.
Since toyocamycin is a nucleotide analog, we examined whether other nucleotide analogs would inhibit the activity of the IRE1α-XBP1 branch. Similar to toyocamycin, sangivamycin and tubercidin, which have adenosine moieties, inhibited ER stress-induced activation of XBP1, as evaluated via XBP1-luciferase assay and RT-PCR analysis. The IC50 values of sangivamycin and tubercidin were 500 nM and 340 nM, respectively. On the other hand, neither 5-aza-2-deoxycytidine, a cytidine analog, nor 5-fluorouridine, a uridine analog, inhibited ER stress-induced activation of XBP1, even at a concentration of over 100 μM (Fig. 2c). These results suggested that the adenosine moiety of toyocamycin is important for its inhibitory activity against XBP1 activation, which supports our hypothesis that toyocamycin may inhibit cofactor binding to IRE1α.
We also examined the anti-tumor activity of toyocamycin against MM, because the IRE1α-XBP1 branch has been reported to be activated in MM cells. MM is a hematological malignancy characterized by the accumulation of clonogenic mature plasma cells in the bone marrow. Terminal differentiation of B lymphocytes to plasma cells requires XBP1 activation [50]. The plasma cells and MM cells must enhance the capacity of the ER in order to produce abundant immunoglobulins and cytokines. Since MM cells are located in the bone marrow, they are thought to exist under hypoxic conditions [51], and abundant and deregulated expression of XBP1 has been detected in these cells [21, 52]. For these reasons, the IRE1α-XBP1 branch is considered to be a therapeutic target for MM cells. Indeed, we showed that XBP1 was constitutively activated in such cells, and toyocamycin suppressed this activation and induced apoptosis. Moreover, toyocamycin showed in vivo anti-tumor activity in an MM xenograft model (at a dose of 0.5 mg/kg twice a week) [42].
Other compounds modulating the IRE1α-XBP1 branch
Beside toyocamycin, several other compounds have been reported to inhibit the IRE1α-XBP1 branch, including 4μ8C, MKC-3946, STF-083010, and APY-29 (Fig. 3). MKC-3946 and 4μ8C were found to inhibit the IRE1α-XBP1 branch in a high-throughput screening using an in vitro FRET-based XBP1 mRNA cleavage assay. While the exact mechanism through which MKC-3946 inhibits IRE1α-mediated XBP1 mRNA cleavage remains unclear, MKC-3946 suppressed XBP1 mRNA splicing without affecting IRE1α phosphorylation in both MM cell lines and an MM tumor xenograft model [53]. 4μ8C was shown to be a noncompetitive inhibitor of the RNase activity of IRE1α, with an IC50 value of 60 nM [54]. Similar to toyocamycin, chemical screening using HT1080 human fibrosarcoma cell lines stably expressing a luciferase-based XBP1 reporter construct identified STF-083010 as an inhibitor against the IRE1α-XBP1 branch. STF-083010 inhibited the RNase activity of IRE1α at 30 μM without affecting the kinase activity of IRE1α, and also inhibited the growth of tumors in a human MM mouse xenograft model (at a dose of 30 mg/kg/week) [55]. Based on all the findings regarding these inhibitors and toyocamycin, the IRE1α-XBP1 branch is a promising therapeutic target for MM.
On the other hand, compounds that selectively activate the IRE1α-XBP1 branch are considered as candidate drugs against diabetes and neurodegenerative diseases. According to an in vitro fluorescence quenching-based screening, quercetin was found to activate the RNase activity of IRE1α and induce XBP1 mRNA splicing [56]. However, the anti-diabetes and anti-neurodegeneration activities of quercetin remain unclear.
References
Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–56.
Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–206.
Korennykh AV, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–93.
Lee KP, et al. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell. 2008;132:89–100.
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32.
Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–9.
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91.
Shen X, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903.
Shi Y, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–509.
Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4.
Schindler AJ, Schekman R. In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proc Natl Acad Sci USA. 2009;106:17775–80.
Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–99.
Ye J, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–64.
Lee E, et al. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–53.
Zhang Y, et al. Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3K and enhances PI(3,4,5)P3 production. PLoS ONE. 2013;8:e80071.
Li Z, et al. Cell-surface GRP78 facilitates colorectal cancer cell migration and invasion. Int J Biochem Cell Biol. 2013;45:987–94.
Delie F, Petignat P, Cohen M. GRP78 protein expression in ovarian cancer patients and perspectives for a drug-targeting approach. J Oncol. 2012;2012:468615.
Lee HK, et al. GRP78 is overexpressed in glioblastomas and regulates glioma cell growth and apoptosis. Neuro Oncol. 2008;10:236–43.
Fujimoto T, et al. Upregulation and overexpression of human X-box binding protein 1 (hXBP-1) gene in primary breast cancers. Breast Cancer. 2003;10:301–6.
Shuda M, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–14.
Munshi NC, et al. Identification of genes modulated in multiple myeloma using genetically identical twin samples. Blood. 2004;103:1799–806.
Romero-Ramirez L, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–7.
Chen X, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508:103–7.
Cullinan SB, et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–209.
Bobrovnikova-Marjon E, et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–95.
Atkins C, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002.
Egawa N, et al. The endoplasmic reticulum stress sensor, ATF6alpha, protects against neurotoxin-induced dopaminergic neuronal death. J Biol Chem. 2011;286:7947–57.
Sado M, et al. Protective effect against Parkinson’s disease-related insults through the activation of XBP1. Brain Res. 2009;1257:16–24.
Zuleta A, Vidal RL, Armentano D, Parsons G, Hetz C. AAV-mediated delivery of the transcription factor XBP1s into the striatum reduces mutant Huntingtin aggregation in a mouse model of Huntington’s disease. Biochem Biophys Res Commun. 2012;420:558–63.
Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61.
Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperon-. 1996;1:109–15.
Xie Q, et al. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601.
Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40.
Zhou Y, et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17:356–65.
Tashiro E, et al. Trierixin, a novel Inhibitor of ER stress-induced XBP1 activation from Streptomyces sp. 1. Taxonomy, fermentation, isolation and biological activities. J Antibiot. 2007;60:547–53.
Futamura Y, et al. Trierixin, a novel Inhibitor of ER stress-induced XBP1 activation from Streptomyces sp. II. structure elucidation. J Antibiot. 2007;60:582–5.
Kawamura T, Tashiro E, Yamamoto K, Shindo K, Imoto M. SAR study of a novel triene-ansamycin group compound, quinotrierixin, and related compounds, as inhibitors of ER stress-induced XBP1 activation. I. Taxonomy, Fermentation, Isolation, Biological Activities and SAR Study. J Antibiot . 2008;61:303–11.
Kawamura T, Tashiro E, Shindo K, Imoto M. SAR study of a novel triene-ansamycin group compound, quinotrierixin, and related compounds, as inhibitors of ER stress-induced XBP1 activation. II. Structure Elucidation. J Antibiot. 2008;61:312–7.
Kakeya H, et al. Cytotrienin A, a novel apoptosis inducer in human leukemia HL-60 cells. J Antibiot. 1997;50:370–2.
Lindqvist L, et al. Inhibition of translation by cytotrienin A-a member of the ansamycin family. RNA. 2010;16:2404–13.
Yamamoto K, Tashiro E, Imoto M. Quinotrierixin inhibited ER stress-induced XBP1 mRNA splicing through inhibition of protein synthesis. Biosci Biotechnol Biochem. 2011;75:284–8.
Ri M, et al. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood. Cancer J. 2012;2:e79.
Nishimura H, Katagiri K, Sato K, Mayama M. Shimaoka N. Toyocamycin, a new anti-candida antibiotics. J Antibiot. 1956;9:60–2.
Tavitian A, Uretsky SC, Acs G. The effect of toyocamycin on cellular RNA synthesis. Biochim Biophys Acta. 1969;179:50–7.
Osada H, Sonoda T, Tsunoda K, Isono K. A new biological role of sangivamycin; inhibition of protein kinases. J Antibiot. 1989;42:102–6.
Osada H, Cui CB, Onose R, Hanaoka F. Screening of cell cycle inhibitors from microbial metabolites by a bioassay using a mouse cdc2 mutant cell line, tsFT210. Bioorg Med Chem. 1997;5:193–203.
Nishioka H, et al. Inhibition of phosphatidylinositol kinase by toyocamycin. J Antibiot. 1990;43:1586–9.
Tirasophon W, Lee K, Callaghan B, Welihinda A, Kaufman RJ. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 2000;14:2725–36.
Zhou J, et al. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci USA. 2006;103:14343–8.
Reimold AM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–7.
Asosingh K, et al. Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematologica. 2005;90:810–7.
Carrasco DR, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11:349–60.
Volkmann K, et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J Biol Chem. 2011;286:12743–55.
Cross BC, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci USA. 2012;109:E869–78.
Papandreou I, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–4.
Wiseman RL, et al. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol Cell. 2010;38:291–304.
Acknowledgements
We wish to thank all past and present colleagues whose names are cited in the references for their contributions. We are especially grateful to Prof. M Imoto for the fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tashiro, E. Screening and identification of inhibitors of endoplasmic reticulum stress-induced activation of the IRE1α-XBP1 branch. J Antibiot 72, 899–905 (2019). https://doi.org/10.1038/s41429-019-0219-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0219-3