Abstract
Bacteriocins hold unprecedented promise as a largely untapped source of antibiotic alternatives in the age of multidrug resistance. Here, we describe the first approach to systematically design variants of a novel AS-48 bacteriocin homologue, which we have termed safencin AS-48, from Bacillus safensis, to gain insights into engineering improved activity of bacteriocins. A library of synthetic peptides in which systematic amino acid substitutions to vary the periodicity and abundance of polar, acidic, aliphatic, and hydrophobic residues were generated for a total of 96 novel peptide variants of a single bacteriocin candidate. Using this method, we identified nine synthetic safencin (syn-safencin) variants with broad and potent antimicrobial activities with minimal inhibitory concentrations (MIC) as low as 250 nM against E. coli, P. aeruginosa, X. axonopodis, and S. pyogenes with minimal cytotoxicity to mammalian cells. It is anticipated that the strategies we have developed will serve as general guides for tuning the specificity of a given natural bacteriocin compound for therapeutic specificity.
Similar content being viewed by others
Introduction
Novel chemical scaffolds for the design of antibiotic compounds have become a priority, as bacterial species have largely become resistant to traditional antibiotics [1]. Antimicrobial peptides (AMPs) represent an unprecedented source of chemical and functional diversity that hold enormous potential for the development of future antibiotics. AMPs have been historically studied owing to two primary roles in nature: defense against infection and niche competition [2]. The AMPs of eukaryotes have been extensively researched for their direct action on pathogens as well as indirect roles as components of the innate immune system. Many of these peptides have conserved structural features, with common amphiphilic and alpha helical domains often serving as scaffolds for the design and optimization of synthetic AMPs [3, 4]. Strategies to improve synthetic AMP activities have generally focused on increasing the targeting affinity for anionic bacterial membranes via incorporation of basic amino acid residues and improving their ability to penetrate lipid membrane domains via incorporation of hydrophobic residues [4]. The general mechanism of these peptides involves the accumulation of the peptide on the bacterial cell surface, with subsequent membrane disruption, leading to cell death [5, 6].
The AMPs of bacteria, bacteriocins, are a group of genetically encoded and ribosomally produced peptides that exist in operons containing the genes necessary for their assembly and export [7, 8]. Although bacteriocins are highly diverse in structure and function, the most fundamental division of bacteriocins, based on structure, is into class I (modified) and class II (unmodified) types [8]. In both cases, bacteriocins are believed to generally undergo the cleavage of a leader sequence from the core peptide domain. In addition to leader sequence cleavage, class I peptides are subject to additional post-translational modifications, including heterocyclization, glycosylation, and head-to-tail circularization [7, 9]. The class I bacteriocin nisin, which has been widely approved for use as a food preservative, is currently being researched for increased activity against infectious bacteria [10, 11]. Nisin is distinguished by posttranslational installation of dehydroalanine and a thioether polycyclic lanthionine bridge [12, 13]. Thiopeptides, a type of class I bacteriocin containing thiazole rings, have been used for the development of antibiotic lead compounds for the treatment of Clostridium difficile infections. Using a naturally occurring thiopeptide as a scaffold, the compound LFF571 was designed by using traditional medicinal chemistry and structure–activity relationship approaches and is now in clinical trials [14, 15]. Despite these significant advances, bacteriocins are still highly underrepresented as template sources for the design of linear AMPs.
Enterocin AS-48 is a class I circular bacteriocin produced by Enterococcus sp. and has been most commonly studied as a possible food preservative [16,17,18]. This bacteriocin is first produced as a prepropeptide consisting of a leader sequence and a propeptide. Subsequent proteolytic cleavage of the leader sequence results in a propeptide that undergoes head to tail macrocyclization to produce the active product [19]. Mature AS-48 consists of five alpha helices, with cationic residues clustered within helices four and five. These residues have been hypothesized as critical for the antimicrobial activity of AS-48 [19,20,21]. However, peptide variants consisting of portions of this region obtained by limited proteolysis or chemical synthesis were not found to retain full antibacterial activity [19, 20, 22]. Here, we describe a novel approach to identifying and verifying portions of AS-48 bacteriocins as scaffolds for the design of synthetic AMPs and their subsequent optimization for charge and hydrophobicity using a peptide library approach.
Materials and methods
Computer models
All 3-D peptide models were predicted using the PEP-FOLD online server [23, 24].
Peptide synthesis and design
Peptides were commercially synthesized by Genscript (Piscataway, NJ). The length of the parent peptide was reduced from 31 to 25 amino acids for ease of library synthesis. The parent peptide was dissolved in DMSO or water to final concentration of 10 mM. This served as a stock from which subsequent peptide dilutions were made for experimental conditions. The library peptides were dissolved in 10% DMSO/water to a final stock concentration of 1 mM. All peptides were synthesized to >95% purity and verified by HPLC and mass spectrometry. Sample data traces of the syn-safencin library parent peptide and library peptide 11 demonstrating purity and composition are provided in the Supplemental Fig. 1. It has been observed that AMPs are enriched for the cationic residues lysine and arginine and the hydrophobic residues phenylalanine and tryptophan [4, 25, 26]. Using this as a guideline we designed a library of 96 peptides consisting of the 25-amino acid parent and 95 optimized variants. Briefly, the 25-amino acid parent peptide was scanned with lysine by replacing the acidic and polar residues to create seven modified peptides. These eight peptides were further modified by scanning the short-chained aliphatic and non-polar amino acids with tryptophan to create 96 unique peptides. A graphical representation of this approach is included in Supplemental Fig. 3.
Bacteria and growth conditions
E. coli BL-21 (Thermo Fischer), P. aeruginosa PAO1 (gift from J. Shrout at University of Notre Dame), and P. syringae (provided by R. Innes, Indiana University) were grown in LB broth Miller (EMD Chemicals, Gibbstown, NJ). GAS M1T1 and MRSA-JKD were grown in Todd Hewitt broth (Neogen Corporation, Lansing, MI). Finally, Xanthomonas axonopodis pathovar Starr and Garces pathovar phaseoli (ATCC 9563) was grown in Nutrient Broth (Sigma-Aldrich Co., St. Louis, MO). P. syringae and X. axonopodis were grown at room temperature. All other bacteria were grown at 37 °C.
MIC determination and peptide screening
Overnight bacterial cultures were diluted to an OD of .01. 90 μL of diluted culture and 10 μL of 10× peptide or vehicle were added to the wells of a 96-well microtiter plate for a final 1× concentration. Bacteria were grown in desired growth conditions in a Synergy H1 Microplate Reader (Biotek, Winooski, VT). For MIC, serial two-fold dilutions of the peptides were used. MIC was determined as the concentration of peptide that prevented overnight growth as indicated by OD 600. For peptide screening, 8 or 4 μM peptide was screened against various bacteria. Peptides that inhibited overnight growth were selected for further study.
Peptide cytotoxicity assays
Eukaryotic cytotoxicity was determined by ethidium homodimer and hemolysis assays. Ethidium homodimer assays were carried out with HaCaT cells in 24-well culture dishes grown to 90% confluency. Medium was aspirated and cells were washed with PBS. Peptide in fresh DMEM was added to the cells at the desired concentration. Cells were incubated with peptide for 16 h. Medium was aspirated and cells were washed with PBS. Cells were incubated in 4 μM ethidium homodimer (Molecular Probes) in PBS for 30 min. Fluorescence was determined by 528 excitation and 617 nm emission and a cutoff value of 590 nm. Saponin (.1%) was then added to each well and incubated for 20 min. The fluorescence was read again. Percent membrane permeabilization was determined by dividing the initial fluorescence by the second fluorescence reading. For hemolysis assays, 100 μL of sheep red blood cells (RBCs) were washed three times in cold PBS (Thermo Fischer). Washed cells were resuspended in 25 mL of PBS. Triton, PBS, or peptide in 10% DMSO/PBS were added to 180 μL of resuspended RBCs and incubated at 37 °C for 1 h. Samples were read at 450 nm. Data was expressed as percent hemolysis by relativizing to the Triton and PBS controls.
Circular dichroism spectroscopy
Peptides were dissolved to a final concentration of 25 μM in the following solvents: 9 mM SDS (Sigma-Aldrich), 50% trifluoroethanol (TFE) (Sigma-Aldrich), and water. For peptide experiments in LPS (Sigma-Aldrich), peptide was dissolved to a final concentration of 5 μM in 5 μM LPS [27, 28]. Using a Jasco Circular Dichroism Spectrometer, samples were scanned from 190 to 250 nm at 20 nm/min in a 2 mm cuvette. Data were averaged over three scans and blanks of the solvent were subtracted from the peptide scans.
Fluorescent microscopy and FACs sorting
Overnight culture of BL-21 E. coli were diluted to a starting OD = 1. 1 mL of cells were washed three times in saline solution. Cells were then resuspended in 1 mL of saline, 70% isopropyl alcohol, or peptide in saline and incubated at 37 °C for 30 min. Cells were pelleted and resuspended in 1 mL of saline with 1.5 μL/mL propidium iodide (PI) (Thermo Fischer) and incubated at room temperature in the dark for 15 min. Samples were pelleted and washed three times with saline. Samples were resuspended in 1 mL of saline. 5 μL of the sample were imaged on a Nikon microscope using the RFP and DIC channels with a 100 and 300 ms exposure times, respectively. For flow cytometry, cells were prepared using the same protocol for microscopy. Cells were suspended in 200 μL of saline. Samples were run on the FACs Aria (BD Biosciences) using the Texas Red channel.
Results
Identification and synthesis of syn-safencin
Previously, using bioinformatics to mine for putative bacteriocins, we identified an AS-48 analog in a newly isolated strain of Bacillus safensis from Vigna radiata seeds, which we have termed safencin AS-48 (Fields, F., unpublished). Amino acid comparison of safencin AS-48 to enterocin AS-48 showed high levels of conservation, including the presence of cationic residues in regions corresponding to helices four and five of the peptide (Supplemental Fig. 2). To determine whether these cationic domains could alone provide antimicrobial activity, we designed a linear synthetic peptide incorporating residues 39–70 of the mature AS-48 peptide from B. safensis, which we henceforth designate as syn (synthetic)-safencin.
Antibacterial and cytotoxic characterization of syn-safencin
Syn-safencin is a 31-amino acid peptide that was synthesized and produced to 95% purity (Genscript). We assessed the antimicrobial activity of syn-safencin on a lab strain of E. coli as well as a known gram-negative pathogen of Vigna radiata, X. axonopodis. Syn-safencin exhibited dose-dependent bacteriostatic activity against E. coli at 16 h post incubation (Fig. 1a). Syn-safencin demonstrated strong bacteriostatic activity against the plant pathogen X. axonopodis, with an MIC of 8 μM (Fig. 1b). We next evaluated general eukaryotic cytotoxicity of syn-safencin. HaCaT cells treated for 16 h showed a slight dose-dependent increase in cytotoxicity; however, even at the highest dose of peptide (32 μM) the percent of cytotoxicity observed (~25%) was similar to vehicle control (17%) (Fig. 1c). However, there was no significant increase in cytotoxicity observed at 8 μM of peptide, the MIC for X. axonopodis. In addition, syn-safencin displayed mild hemolytic activity at the concentrations tested, suggesting its activity as a membrane lysin (Fig. 1d).
Antimicrobial activity and eukaryotic cytotoxicity of syn-safencin. a Antibacterial activity of syn-safencin on E. coli as determined by OD 600 reading after 16 h of growth. b MIC determination of syn-safencin on X. axonopodis as determined by OD 600 reading after 16 h of growth. c Ethidium homodimer measurements of cytotoxicity of syn-safencin on HaCaT cells after 16 h of treatment. d Hemolysis assays of syn-safencin-treated sheep RBCs. p-values were determined via one-way ANOVA and Tukey post hoc analysis. Asterisk indicates a p < .05. ns indicates a p > .05. All data represents the average of three biological replicates (n = 3). Error bars represent the standard error of the mean
Secondary structure analysis and method of action of syn-safencin
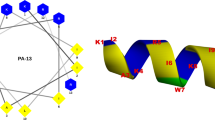
Using secondary structure prediction software, syn-safencin was predicted to be an alpha helical peptide consisting of a hydrophobic and a cationic face, suggesting that our artificial peptide preserves the common amphipathic, helical, and cationic features of many linear AMPs [3, 23, 24] (Fig. 2a). To support the modeling data, circular dichroism was used to assess the secondary structure of syn-safencin. Many cationic AMPs have been observed to adopt an alpha helix in membrane mimicking environments such as SDS and TFE, while having a random coil conformation in aqueous environments [22, 28, 29]. CD analysis of syn-safencin showed a shift from a random coil conformation in an aqueous environment (ddH2O) to an alpha helical signal in both 9 mM SDS and 50% TFE (Fig. 2b–c). To determine whether the peptide could adopt an alpha helical signature in the presence of bacterial membrane components, we performed CD analysis of syn-safencin in the presence of purified LPS micelles. Similar to earlier spectra of the peptide in the presence of defined membrane mimics, an alpha helical signal was observed in the presence of LPS micelles, suggesting that bacterial LPS is likely a major target for the peptide (Fig. 2d).
Syn-safencin secondary structure. a Syn-safencin PEP-FOLD secondary structure prediction. Hydrophobic, cationic, and anionic residues are orange, blue, or red, respectively. b CD spectroscopy of 25 μM syn-safencin in 9 mM SDS, c 50% TFE, and d 5 μM syn-safencin in 5 μM LPS. Gray lines are peptide dissolved in ultrapure water. Black lines are peptide dissolved in water with a membrane mimic. Data represents the average of three scans (n = 3)
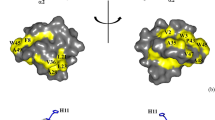
To assess whether syn-safencin could directly permeabilize bacterial membranes, PI staining was used to evaluate overall membrane permeability in the presence of peptide treatment. As observed by fluorescence microscopy, peptide-treated cells showed positive staining for PI (Fig. 3c–e) similar to the isopropyl alcohol control (Fig. 3b). In addition, E. coli cells appeared to become elongated in the presence of the peptide (Fig. 3e). This elongation phenotype has been observed in E. coli as a response to membrane stress caused by cationic AMPs [30]. FACs sorting of peptide-treated cells confirmed an increase in PI-positive cell count in all peptide-treated groups (Fig. 3f). Taken together, our results demonstrate that we can successfully design a synthetic peptide comprising a specific minimal domain within the full-length AS-48 bacteriocin that retains antimicrobial activity. Furthermore, our results predict that syn-safencin is a cationic, amphipathic, helical, AMP that exerts antibacterial activity by permeabilizing the outer membrane via interaction with LPS.
Propidium iodide uptake into syn-safencin-treated E. coli. a Saline and b isopropyl alcohol (IP) were used as negative and positive controls, respectively. E. coli were treated with c 32 μM, d 16 μM, or e 8 μM syn-safencin. Images are representative of three biological replicates (n = 3). f FACs sorting analysis of syn-safencin-treated E. coli. Saline (brown) and IP (black) are used as negative and positive controls, respectively. Cells were treated with either 32 μM (red), 16 μM (blue), 8 μM (purple), 4 μM (green), or 2 μM (yellow) syn-safencin
Peptide library design and characterization
We next set about to take a systematic approach to determine whether amino acid variants of syn-safencin could be designed for improved activity. We designed a peptide library based on the original peptide with amino acid changes to vary the hydrophobicity and charge of the original peptide (Supplemental Fig. 3). For library construction, an additional six amino acids were removed, to create a 25 residue syn-safencin template from which variants were designed. Seven peptide variants were created by substituting lysine for acidic and polar amino acids. Next, the parent peptide and the seven variants served as templates for optimization of overall hydrophobicity. Tryptophan was substituted for nonpolar amino acids and short-chained amino acids. In total, 96 syn-safencin variants were created. These peptides were screened at 8 μM against E. coli, S. pyogenes, S. aureus, P. syringae, and P. aeruginosa. Peptides were screened against X. axonopodis at 4 μM. From these screens, we selected nine peptides that were broadly active against multiple bacteria (Supplemental Table 1). All peptides exhibited low to no cytotoxicity after overnight incubation on HaCaT cells (Supplemental Fig. 4). However, peptides 52, 90, 91, 93, and 94 exhibited low levels of hemolysis at higher concentrations (Supplemental Fig. 5). Peptides 20, 60, 92, and 96 showed no significant changes in hemolysis when compared to the PBS control (Supplemental Fig. 5).
Secondary structure predictions using PEP-FOLD software confirmed that each of the peptide variants retained a helical structure; however, all peptides with the G18W replacement existed as a full alpha helix, while peptides without this mutation existed as an N-terminal coil with a C-terminal helix (Fig. 4a, b). The secondary structure predictions of all syn-safencin peptide variants revealed a clustering of the hydrophobic residues on one side of the helix with most of the charged residues localizing to the other side of the helix (Fig. 4a). To assess if these peptides were more active than the parent peptide (syn-safencin), additional MIC assays were conducted on E. coli, X. axonopodis, P. aeruginosa, and S. pyogenes. MICs as low as 250 nM were observed for the optimized synthetic variant peptides, making these optimized peptides more potent than the original syn-safencin peptide (Fig. 4c).
Characterization of library optimized syn-safencin variants. a Secondary structure predictions of peptide library hits. Hydrophobic, cationic, and anionic residues are orange, blue, or red, respectively. b Alignments of peptide library hits to the 25 residue syn-safencin parent peptide. Residue changes are highlighted in red. c MIC table of peptide library hits against a variety of organisms. Data are representative of three biological replicates (n = 3)
Discussion
We have described a general strategy for the identification and design of linear synthetic peptide variants of an AS-48-like bacteriocin using a systematic peptide library approach. Our approach serves as a general guideline for the optimization and refinement of bacteriocin design for possible therapeutic applications. Previous studies have hypothesized that the cationic stretch of amino acids in helices four and five of enterocin AS-48 may serve as its putative membrane interacting region [19,20,21,22]. However, peptide fragments consisting of only these residues did not exhibit any antimicrobial activity in previous reports [20]. In our study, we have designed a new peptide, syn-safencin, which includes the cationic domain that spans helices four and five homologous to enterocin AS-48, and our data confirm that this critical domain alone can confer antimicrobial activity. We demonstrate that the incorporation of cationic residues and preservation of hydrophobic amino acids are essential to the retention of antimicrobial activity in our linear AS-48 variants (Table 1). Linear AS-48 variants consisting of the membrane interacting region and W70 retained antimicrobial activity as exhibited in both syn-safencin and the linearized variants of enterocin AS-48 (Table 1) [22]. However, when I59, V67, and W70 are exchanged, as is the case in AS-48 peptide 49–69, activity is lost (Table 1) [21]. These data indicate that hydrophobic and cationic residues within the putative membrane-interacting region of helices four and five are critical for the antimicrobial activity of our designed linear AS-48 variants.
Our CD data show that syn-safencin exists as a random coil in an aqueous environment, while adopting a helical conformation in the presence of membrane mimics, including LPS (Fig. 2b–d). This structural preference for membrane mimics has been observed for many membrane active AMPs [28, 29, 31]. Our CD studies in the presence of E. coli LPS offer a more specific model of membrane disruption in which syn-safencin is electrostatically attracted to the negatively charged phospholipid head groups allowing for its hydrophobic residues to interact with lipid moieties.
These general structural features of syn-safencin allow for the optimization of antibacterial activity using a systematic amino acid substitution approach (Supplemental Fig. 3). Synthetic AMP variants can be enriched for cationic and hydrophobic residues; therefore, we reasoned that modifying the charge and hydrophobicity of syn-safencin could increase the activity of the peptide [31,32,33]. As observed in our screen, we reduced the MIC of syn-safencin against target organisms using this heuristic approach. One common feature of the optimized peptides was the substitution of glycine for tryptophan (Fig. 4b). Glycine is a commonly known helix breaker and is naturally located in the turn regions of AS-48 [34]. Substitution of glycine for tryptophan extends the helix, increasing the overall helical propensity of the peptide, which may be correlated to the increased antimicrobial activity that we observed in these variants [6]. Increasing the positive charge and localizing it to one face of the helical peptide was achieved through substitution of glutamic acid for lysine (Fig. 4b). Increased antimicrobial activity of these peptides may be due to the increased positive charge on the hydrophilic face of the helix [3]. Secondary structure models of these optimized peptides show an idealized amphipathic nature (Fig. 4a). This idealization of the amphiphilic helix has been previously shown to contribute to increased antimicrobial activity [6, 31].
In conclusion, we have demonstrated the successful synthesis of an AS-48-like bacteriocin variant that can be synthesized efficiently and retains antimicrobial activity. Furthermore, we describe a systematic library approach to optimize the design and efficacy of our bacteriocin variants. We show that several peptide candidates designed using our approach exhibit potent activity against a broad spectrum of target microorganisms. The approach described herein therefore represents a general strategy through which linear variants of many circular bacteriocins can be modified for increased activity and future use as direct therapeutic compounds [8, 14]. We are currently pursuing additional studies to determine the efficacy, stability, and toxicity of these peptides in vivo.
References
CDC. Antibiotic resistance threats in the United States. United States Department of Health and Human Services, Centers for Disease Control and Prevention. 2013 (http://www.cdc.gov/drugresistance/threat-report-2013/).
Ageitos JM, Sánchez-Pérez A, Calo-Mata P, Villa TG. Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol. 2017;133:117–38.
Uggerhøj LE, et al. Rational design of alpha-helical antimicrobial peptides: do’s and don’ts. ChemBioChem. 2015;16:242–53.
Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2012;11:37–51.
Lv Y, et al. Antimicrobial properties and membrane-active mechanism of a potential α-helical antimicrobial derived from cathelicidin PMAP-36. PLoS ONE. 2014;9:e86364.
Ong ZY, Wiradharma N, Yang YY. Strategies employed in the design and optimization of synthetic antimicrobial peptide amphiphiles with enhanced therapeutic potentials. Adv Drug Deliv Rev. 2014;78:28–45.
Alvarez-Sieiro P, Montalbán-López M, Mu D, Kuipers OP. Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biotechnol. 2016;100:2939–51.
Cotter PD, Ross RP, Hill C. Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11:95–105.
Arnison PG, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30:108–60.
Field D, et al. A bioengineered nisin derivative to control biofilms of Staphylococcus pseudintermedius. PLoS ONE. 2015;10:e0119684.
Field D, Cotter PD, Hill C, Ross RP. Bioengineering lantibiotics for therapeutic success. Front Microbiol. 2015;6:1–6.
Murinda SE, Rashid KA, Roberts RF. In vitro assessment of the cytotoxicity of nisin, pediocin, and selected colicins on simian virus 40-transfected human colon and Vero monkey kidney cells with trypan blue staining viability assays. J Food Prot. 2003;66:847–53.
Field D, Cotter PD, Ross RP, Hill C. Bioengineering of the model lantibiotic nisin. Bioengineered. 2015;5979:37–41.
Lamarche MJ, et al. Discovery of LFF571: an investigational agent for Clostridium difficile infection. J Med Chem. 2012;55:2376–87.
Mullane K, et al. Multicenter, randomized clinical trial to compare the safety and efficacy of LFF571 and vancomycin for Clostridium difficile infections. Antimicrob Agents Chemother. 2015;59:1435–40.
Burgos MJG, Aguayo MCL, Pulido RP, Gálvez A, López RL. Inactivation of Staphylococcus aureus in oat and soya drinks by enterocin AS-48 in combination with other antimicrobials. J Food Sci. 2015;80:2030–4.
Caballero Gómez N, Abriouel H, José Grande M, Pérez Pulido R, Gálvez A. Combined treatments of enterocin AS-48 with biocides to improve the inactivation of methicillin-sensitive and methicillin-resistant Staphylococcus aureus planktonic and sessile cells. Int J Food Microbiol. 2013;163:96–100.
Gómez NC, Abriouel H, Grande J, Pulido RP, Gálvez A. Effect of enterocin AS-48 in combination with biocides on planktonic and sessile Listeria monocytogenes. Food Microbiol. 2012;30:51–8.
Sánchez-Hidalgo M, et al. AS-48 bacteriocin: close to perfection. Cell Mol Life Sci. 2011;68:2845–57.
Montalbán-López M, Martínez-Bueno M, Valdivia E, Maqueda M. Expression of linear permutated variants from circular enterocin AS-48. Biochimie. 2011;93:549–55.
Angeles Jiménez M, Barrachi-Saccilotto AC, Valdivia E, Maqueda M, Rico M. Design, NMR characterization and activity of a 21-residue peptide fragment of bacteriocin AS-48 containing its putative membrane interacting region. J Pept Sci. 2005;11:29–36.
Montalbán-López M, et al. Characterization of linear forms of the circular enterocin AS-48 obtained by limited proteolysis. FEBS Lett. 2008;582:3237–42.
Thévenet P, et al. PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012;40:W288–93.
Maupetit J, Derreumaux P, Tuffery P. PEP-FOLD: an online resource for de novo peptide structure prediction. Nucleic Acids Res. 2009;37:W498–503.
Fimland G, Eijsink VGH, Nissen-Meyer J. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry. 2002;41:9508–15.
Nguyen, LT, et al. Serum stabilities of short tryptophan-and arginine-rich antimicrobial peptide analogs. PLoS ONE. 2010;5:1–8.
Aurell CA, Wistrom AO. Critical aggregation concentrations of gram-negative bacterial lipopolysaccharides (LPS). Biochem Biophys Res Commun. 1998;253:119–23.
Avitabile C, D’Andrea LD, Romanelli A. Circular dichroism studies on the interactions of antimicrobial peptides with bacterial cells. Sci Rep. 2014;4:4293.
Sreerama N, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem. 2000;287:252–60.
Yadavalli SS, et al. Antimicrobial peptides trigger a division block in Escherichia coli through stimulation of a signalling system. Nat Commun. 2016;7:12340.
Deslouches B, et al. Rational design of engineered cationic antimicrobial peptides consisting exclusively of arginine and tryptophan, and their activity against multidrug-resistant pathogens. Antimicrob Agents Chemother. 2013;57:2511–21.
Mikut R, et al. Improving short antimicrobial peptides despite elusive rules for activity. BBA Biomembr. 2016;1858:1024–33.
Mojsoska B, Carretero G, Larsen S, Mateiu RV, Jenssen H. Peptoids successfully inhibit the growth of gram negative E. coli causing substantial membrane damage. Sci Rep. 2017;7:42332.
Cebrián R, et al. The bacteriocin AS-48 requires dimer dissociation followed by hydrophobic interactions with the membrane for antibacterial activity. J Struct Biol. 2015;190:162–72.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Fields, F.R., Carothers, K.E., Balsara, R.D. et al. Rational design of syn-safencin, a novel linear antimicrobial peptide derived from the circular bacteriocin safencin AS-48. J Antibiot 71, 592–600 (2018). https://doi.org/10.1038/s41429-018-0032-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-018-0032-4