Abstract
Inspired by ocean organisms, scientists have been developing adhesives for application in the marine environment. However, water and high salinity, which not only weaken the interfacial bonding by the hydration layer but also induce the deterioration of adhesives by erosion, swelling, hydrolysis, or plasticization, are detrimental to adhesion, resulting in specific challenges in the development of under-seawater adhesives. In this focus review, current adhesives that are capable of macroscopic adhesion in seawater were summarized. The design strategies and performance of these adhesives were reviewed based on their bonding methods. Finally, some future research directions and perspectives for under-seawater adhesives were discussed.
Similar content being viewed by others
Introduction
The application of adhesives plays a crucial role in the exploration and utilization of marine resources. Many marine applications, such as underwater sonar equipment, protective/antifouling layer coatings, and offshore structures, require under-seawater adhesives [1,2,3]. Early studies in this area mainly focused on the effect of seawater on the strength aging of epoxy-based and polyurethane-based adhesives [4, 5]. In these studies, bonding joints were generally first prepared in air and then immersed in (artificial) seawater to study their long-term stabilities. However, with the development of science and technology, applications in some emerging fields require adhesives to be directly applied under-seawater, for example, in the case of under-seawater repair, marine cultivation, ocean animal health care, electronic devices, marine robots, and so on (Fig. 1) [6, 7].
However, achieving strong interfacial bonding in seawater is challenging [8,9,10]. First, water and hydrated ions are strongly associated atop the surface of the substrate in seawater and impede the molecular contact of the adhesive and substrate. Second, the high-ionic strength of seawater (Table 1) dramatically weakens interfacial interactions, such as electrostatic interactions and dispersing forces, which prevents molecular-level bonding between the adhesive and the substrate [11,12,13,14]. Moreover, water droplets can be trapped at the interface, which reduces the real contact area, and such a phenomenon is particularly pronounced for soft tape-type adhesives [15,16,17]. In addition, the long-term exposure of submersed bonded joints results in the diffusion of water and salt into the adhesive, causing the swelling, erosion, degradation, or hydrolysis of adhesives, consequently leading to adhesive/cohesive failure. These effects become more significant in real applications due to the nonstatic conditions of the marine environment. For example, variations in temperature and hydraulic pressure and the scour of seawater are particularly detrimental to adhesion [7, 18, 19]. Notably, compared with adhesion under pure water, adhesion under-seawater is more difficult; the high concentration of salt ions in seawater further increases the difficulty of interfacial bonding and accelerates the deterioration of existing adhesion. Therefore, the development of adhesives used in seawater remains a huge challenge.
In contrast to the failure of man-made adhesives in water, successful underwater adhesives have been wonderfully demonstrated in nature [10, 20, 21]. Distinguished examples include mussels and barnacles, which use adhesive proteins to attach to wet rocks to resist the scour of seawater, and octopuses, whose arms have suckers that allow them to capture prey in the sea [22, 23]. Inspired by these unique adhesion strategies, the development of underwater adhesives has come a long way in the past decade. To date, various underwater adhesives have been fabricated by using diverse strategies, which have been summarized in several excellent reviews [24,25,26,27,28,29,30]. Nevertheless, few studies have been conducted that focus on their adhesive performance in seawater, and a review of the related topic has yet to be produced.
In this focus review, I summarized the currently reported adhesives that can directly adhere to substrates under (artificial) seawater/high-salinity water. Based on their bonding methods, the adhesives were classified into glue-type and tape-type adhesives. An overview of the development and performance of typical examples was provided. In addition, some perspectives for under-seawater adhesives were discussed. It should be mentioned that this paper only reviewed the adhesives that were applied on a submersed substrate and cured in seawater/saline water without a drying process in air.
Under-seawater adhesives
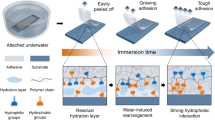
Similar to underwater adhesion, achieving strong under-seawater adhesion also requires three steps. The first and most important step is the removal of the hydration and salt ion layer of the substrate. Recent studies have found that cationic groups can clear surfaces of bound salts and that hydrophobic groups can breakdown the hydration layer to “dry” the underwater surface, making way for adhesion [13, 30, 31]. In addition, using water-absorbing fillers and pattern surfaces for water drainage has also been proven to effectively remove surface water [17, 32, 33]. Following the breakdown of the hydration layer, strong interfacial bonding should be achieved. Since the target adherents used in seawater are usually metals, glass, plastics, etc., noncovalent interactions (hydrogen bonds, electrostatic interactions, hydrophobic interactions, ion-dipole interactions, etc.) and physical suction are the general adhesion mechanisms [29, 34,35,36]. The other key to strong underwater adhesion is the tough mechanical strength of bulk adhesives, which prevents fast crack propagation during the debonding process. Particularly for under-seawater adhesives, adhesive joints are generally sustained by constant loading originating from the complex dynamic environment in the ocean. Therefore, antifatigue properties are essential for long-term adhesion. The basic design principle for toughening is introducing dissipation energy systems in the polymer network by using, for example, dynamic bonds or multiple networks [37,38,39]. In addition, due to the harsh environment in the ocean, adhesives are required to possess anti-erosion and antiswelling properties for long-term stability.
Based on their bonding method, the current under-seawater adhesives can be classified into glue-type and tape-type adhesives. The former is a liquid that needs to be solidified to glue the joint, whereas the latter is a soft solid material that can directly adhere to the substrate. The following sections review the current adhesives that can be used in artificial seawater or water with high salinity in terms of glue and tape systems.
Glue-type adhesives
Glues are a kind of liquid adhesive that require a solidification process to achieve adhesion. The current under-seawater glue mainly includes organic solvent-based polymer glue and water-borne coacervate glue. In general, glue is applied between two adherents that are immersed in seawater. Then, the joint is maintained in seawater for a particular time (hours to days) under external pressure to allow the glue to fully cure. The curing processes are mainly based on the polymerization of monomers and crosslinking of polymers through the formation of chemical bonds and/or physical interactions. Currently reported glue-type under-seawater adhesives and their adhesion performances are listed in Table 2.
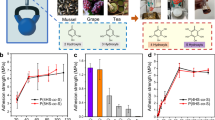
Catechol-based polymer solutions are typical glues that have been widely studied since the catechol group has strong adhesion to diverse substrates and high reactivity that can easily facilitate crosslinking [40,41,42]. Although various catechol-based copolymers have been synthesized and exhibited excellent adhesion on dry substrates, their adhesion performance in seawater or saline water is rarely studied [43, 44]. A pioneering study was reported by White and Wilker in 2011 [32]. They synthesized three-component copolymers bearing cationic, catechol, and benzene groups (Fig. 2a). They dissolved the polymers in a chloroform/methanol mixture and then applied the solutions onto an aluminum substrate immersed in artificial seawater. Due to its higher density compared to that of water, the glue did not float up and off the substrate. Then, a second aluminum sheet was placed on the first sheet and allowed to cure in water for 24 h. The test results showed that the adhesive strength of the polymer glues in seawater first increased and then decreased with increasing cationic content. They explained that the glue with the higher cationic fraction has better surface wetting ability based on the contact angle data, which contributed to improved adhesion. While introducing too much charge (above ~11%) caused strong cohesion but not adhesion, in my view, the weak adhesion may have been caused by the increase in the hydrophilicity of the polymer.
Organic solvent-based polymer glue. a Copolymers bearing cationic, catechol, and benzene residues synthesized by Wilker’s group [32]. b Adhesive copolymers possessing three types of phenolic groups (phenol, catechol, and gallol) and their under-seawater adhesiveness [46]. c Catechol-based alternating sequential copolymer synthesized by Sha et al. [47]. d Catechol-based copolymers with different polarities and their adhesion performance in seawater [49]
Furthermore, North et al. synthesized a series of poly[(3,4-dihydroxystyrene)-co-styrene] polymers with different molecular weights and studied their under-seawater adhesion [45]. The adhesive strength in artificial seawater was found to first increase and then decrease with an increasing molecular weight of the polymer. The glue showed a highest adhesive strength of ~2.5 MPa at a molecular weight of ~85,000 g/mol. In glue systems, a low molecular weight is beneficial for the wetting behavior of the solution, while a high molecular weight can enhance the cohesion of the glue. Therefore, there is an optimum molecular weight for achieving the best adhesion performance.
Zhan et al. studied the influence of the number of hydroxyl substituents on the benzene ring on underwater adhesion [46]. Copolymers bearing three types of phenolic groups (phenol, catechol, and gallol) were synthesized and dissolved in a chloroform/methanol mixture (Fig. 2b). The gallol-based copolymer exhibited the strongest adhesiveness compared to the other two polymers under all tested environments (in air, water, and seawater). The results suggested that the tridentate structure has a stronger underwater interfacial bonding ability than the bidentate and monodentate structures.
Sha et al. synthesized a mussel-inspired alternating copolymer by using click chemistry [47]. In their strategy, dopamine and 2,2-bis(4-glycidyloxyphenyl) propane (BGOP) were copolymerized via an epoxy-amino click reaction (Fig. 2c). Compared with introducing the vinyl group into the catechol group, directly using dopamine is much simpler. Moreover, the click reaction is fast, almost quantitative, and allows polymers with high catechol contents and regular sequences to be obtained. To test its adhesion ability, the polymer was dissolved in a chloroform/methanol mixture to obtain a glue adhesive. They found that the introduced polar groups and rigid bisphenol A structures in the polymer enhanced the cohesion of the adhesive, while the high content of the catechol group provided strong adhesion. As a result, the copolymer showed high adhesiveness under both dry and seawater conditions. This work demonstrated a facile strategy for designing catechol-functionalized copolymers with controlled sequences; however, unfortunately, this work did not study the sequence effect on adhesion performance, which is critical for our understanding of structure-property relationships.
In addition to the catechol group, the backbones of polymers were investigated by Li et al. [48]. Inspired by mussel foot proteins, they raised the question of the relationship between the polarity of the polymer backbone and polymer underwater adhesion. To answer this question, four polymers with similar catechol contents and molecular weights but different backbones were synthesized (Fig. 2d) [49]. The under-seawater adhesion test results showed that the polymer with the higher polarity had stronger adhesiveness. According to my understanding, this phenomenon may have been caused by the modulus difference of the cured adhesives. After curing in seawater, the adhesive film of the low-polarity polymer was more rigid than that of the high-polarity polymer. In general, a rigid adhesive has low resistance against crack growth and exhibits poor adhesive strength.
Since glues based on hydrophobic polymers usually require an organic solvent, which may be harmful to organismic health and the environment, water-borne glues have received much attention in recent years. For example, Zheng et al. developed a polymer glue possessing rapid, strong, long-lasting adhesion and ionic conductivity in water and seawater environments [7]. In their strategy, they synthesized a hydrophobic polymer bearing benzene and nitrogen heterocyclic groups and dissolved it in a water-soluble ionic liquid (Fig. 3a). When injecting the polymer solution into water, the fast water exchange triggered the precipitation of the hydrophobic polymer, resulting in rapid adhesion. With increasing immersion time, the glue was further self-strengthened through the formation of strong hydrophobic interactions, which led to an increase in adhesive strength. For example, the instant under-seawater adhesive strength on glass was ~100 kPa, while it increased to ~400 kPa after 7 days of soaking. In addition, they found that there was ~44.38% ionic liquid retained in the adhesive even after long-term soaking, which provided considerable ionic conductivity and allowed the adhesive to work as an in situ strain sensor.
Water-borne coacervate glue. a Precipitation of a triazole-based polymer in water and its adhesion performance in different substrates and solutions [7]. b Design strategy of a polymer with a cation-methylene-phenyl motif inspired by the protein of membraneless organelles [18]. c Ionic complex-based adhesives fabricated by the polymerization of 4-AS initiated by zwitterions [51]
Zhu et al. proposed a strategy to synthesize a water-borne polymer adhesive inspired by the protein of membraneless organelles [18]. In their study, they synthesized a polymer with a cation–aromatic sequence by using a monomer with a cation-methylene-phenyl (C-M-P) motif (Fig. 3b). The obtained aqueous polymer solution formed coacervates through the addition of NaCl because the high-ionic strength of the solution reduced the electrostatic repulsion between cationic groups but enhanced the interchain cation-π interactions [30, 50]. They further synthesized polymers by the copolymerization of a C-M-P monomer and postcurable monomers and tested their adhesion performance in aqueous conditions. The results showed that the copolymer glue exhibited outstanding adhesion in normal saline water and seawater. For example, a copolymer obtained by copolymerizing the C-M-P monomer with a curable ionic liquid monomer was capable of gluing glass together in a simulated deep-sea environment (3 °C, oxygen-free, and dark) after overnight curing (12 h). In contrast with the difficulty of linear sequence-controlled copolymer synthesis, this work provides a simple strategy to develop polymers with sequential cation-π motifs.
One of the shortcomings of glue adhesives is the long-term curing process. To achieve strong underwater adhesion in a short time, Cui et al. developed a water-borne glue system by utilizing the rapid polymerization of 4-aminostyrene (4-AS) in the presence of an acidic polyanion (Fig. 3c) [51]. In general, pure 4-AS is difficult to polymerize because the reactivity of the vinyl group is inhibited by the charge density transfer from the amino group to the conjugated double bond. However, in the presence of 4-AS salts, 4-AS can be spontaneously polymerized via the zwitterionic mechanism. Based on this phenomenon, they prepared two aqueous solutions, namely, a polyacrylic acid (PAAc) solution and a 4-AS solution. When mixing them, PAAc not only provided acidic conditions for the polymerization of 4-AS but also formed a complex with P(4-AS) through the formation of multiple ionic bonds. As a result, a bright-yellow sticky putty was immediately formed, and it could be fully cured within 20 min to glue diverse substrates in various environments (water, seawater, oil, etc.). In addition, this adhesive could maintain strong adhesion for 6 months.
Recently, Peng et al. developed a coacervate glue that exhibited instant, robust, and repeatable underwater adhesion [52]. The reported coacervate was formed by mixing tannic acid and poly(ethylene glycol)77-b-poly(propylene glycol)29-b-poly(ethylene glycol)77 (F68) aqueous solutions. The abundant gallol group on TA provided strong interfacial adhesion, while the hydrogen bonds between TA and the polymer and the hydrophobic core of F68 micelles facilitated tough cohesion. As a result, the coacervate showed robust and instant adhesion in water as well as in a NaCl solution (0.1–1.0 M). It also displayed excellent repeatability up to 1000 cycles, which is seldom tested in other adhesive systems. Moreover, the biological activities of TA endowed the adhesive with anticancer and antibacterial properties.
Tape-type adhesives
Soft solid materials that can directly adhere to substrates are regarded as tapes. Compared with glues, it is more challenging for tapes to achieve strong under-seawater adhesion. This is because, on the one hand, wetting the surface and breaking down the hydration layer with crosslinked polymers is much more difficult than with unconfined dissolved polymers. On the other hand, water droplets can be easily trapped at the interface, which decreases the real contact area and induces crack defects. To tackle this dilemma, several strategies have been proposed recently, inspired by natural underwater adhesion mechanisms. The tape-type under-seawater adhesives and their adhesion performances are listed in Table 3.
Niu et al. developed an elastomer that exhibited high adhesion in water and seawater conditions [53]. The adhesive was synthesized by the copolymerization of butyl acrylate and acrylic acid (Fig. 4a). After adjusting the monomer ratio to balance the adhesion and cohesion, they optimized the viscoelastic properties of the elastomer so that it achieved the best adhesion performance in seawater. The adhesive strength of the elastomer was in the range of 120–150 kPa depending on the substrates. In addition, the adhesion of the elastomer was reversible due to physical interfacial interactions.
Tape-type under-seawater adhesives. a Rheological behavior of P(BA-co-AAc) elastomers and their underwater adhesiveness [53]. b Schematic illustration of the structure of a poly(ionic liquid) adhesive and its adhesion performance in various solutions [54]. c Schematic illustration of the preparation and structure of organohydrogels and their adhesiveness in seawater [58]
In addition to this hydrophobic elastomer, Yu and Wu developed a hydrophobic ionic liquid gel by using a water-insoluble anion (Fig. 4b) [54]. In their strategy, they first prepared two ionic liquids with the same hydrophobic anion, one being butyltrimethylammonium bis(trifluoromethanesulfonyl) imide ([N4111][TFSI]), working as solvent, the other one being [2-(methacryloyloxy)ethyl]trimethylammonium bis(trifluoromethanesulfonyl) imide ([MATAC][TFSI]) monomer. After one-pot polymerization, a viscoelastic ionogel was fabricated. The ionogel did not swell in water due to its hydrophobicity and exhibited various properties, including optical transparency, tunable mechanical properties, self-healing ability, underwater adhesiveness, conductivity, and 3D printability.
Compared with the hydrophobic elastomer/gel, it is more difficult for a hydrogel to achieve underwater adhesion due to its hydrophilic and highly swollen polymer network. To effectively breakdown the hydration layer and suppress the swelling ratio to improve adhesion, introducing hydrophobic functional groups into hydrogels has been proven to be an effective approach [16, 55]. In this strategy, gels are usually fabricated in a water-miscible organic solvent, followed by a solvent exchange process to obtain hydrogels. For example, Liu et al. copolymerized a hydrophobic and anionic monomer in DMSO and obtained an organogel [56, 57]. They found that the as-prepared organogel could adhere to diverse substrates under various solvents, including water, seawater, and oil, during the exchange of solvent. In the case of adhesion in aqueous conditions, the water diffused into the gel and replaced DMSO, resulting in the dehydration of the surface and the aggregation of hydrophobic groups, which enhanced both adhesion and cohesion. After 24 h of immersion, the adhesive strength reached ~120 kPa.
Zhang et al. developed an organohydrogel with hydrophobic and hydrophilic heteronetwork by the in situ emulsion polymerization of oleophilic and zwitterionic monomers (Fig. 4c) [58]. In the organohydrogel, the oleophilic and hydrophilic polymer chains were spatially restrained in the interpenetrating heteronetwork, which led to antiswelling behavior in both water and oil. The authors further found that the organohydrogel exhibited under-seawater adhesion to various substrates after 5 min of contact. They believed that the synergetic interactions at the interface, including electrostatic and hydrophobic interactions, facilitated the adhesion of the gel. In my understanding, the uneven surface resulting from the mismatch of the swelling degree of the hydrophilic and hydrophobic domains may also have played a key role in facilitating the adhesion of the gel. The concavity of the hydrophobic domains may have accelerated the interfacial water drainage in this system.
Many solid surfaces, including rocks, glass, and metals, are negatively charged in the marine environment [11]. Therefore, a facile strategy using electrostatic interactions as a bonding mechanism would be effective on these surfaces. However, the electrostatic interactions between oppositely charged surfaces in high-ionic-strength environments such as seawater are normally weakened due to the Debye screening effect [11]. To tackle this dilemma, specialized protein sequences have been obtained through evolution in biological systems. Cationic and aromatic amino acids are always adjacent to each other in many adhesive proteins, such as mussel foot proteins, barnacle cement proteins, and coronavirus spike proteins [59,60,61]. Such a specific characteristic enables the proteins to adhere to negatively charged surfaces through electrostatic interactions in a saline environment, providing a design model for developing marine adhesives. However, sequence-controlled polymerization is still a central challenge in polymer chemistry [62].
Recently, our group discovered that copolymers with adjacent cation–aromatic sequences can be synthesized through simple free-radical polymerization at equimolar ratios [30, 63]. It was found that the polymerization behavior of cationic and aromatic monomers in precursor solution highly depends on their vinyl groups, monomer concentration, and solvent. When the cationic and aromatic monomers have the same double bond, at high concentrations and with DMSO as the solvent, the reactivity ratios (r) of the cationic and aromatic monomers are close to 1, namely, they show ideal random copolymerization (Fig. 5a) [63]. In this case, the resulting copolymer has an adjacent-rich sequence, and it is water soluble, although this copolymer has 50% hydrophobic monomers. If it lacks one of these three preconditions, a random copolymer with only one component-rich sequence is obtained. Such a polymer cannot dissolve in water.
Under-seawater adhesive hydrogels with adjacent cationic and aromatic sequences [30, 63]. a Free-radical polymerization for synthesizing copolymers with different cation-π sequences by using DMSO and dimethyl sulfate (DMS). b Digital photo of P(cation-adj-π) and P(cation-co-π) hydrogels equilibrated in 0.7 M NaCl solution and their mechanical properties. c Under-seawater adhesion performance of P(ATAC-adj-PEA) and P(ATAC-co-PEA) hydrogels. ATAC: 2-(acryloyloxy)ethyl trimethylammonium chloride; PEA: 2-phenoxyethyl acrylate. d Photographs showing a P(cation-adj-π) gel adhered to a glass block and the block being lifted out of seawater
By using one-pot free-radical copolymerization, copolymers with different sequences can be synthesized on a large scale, meeting the requirements for material fabrication and studies. For example, due to their different monomer sequences, P(cation-adj-π) and P(cation-co-π) hydrogels showed completely different macroappearances in water and saltwater (Fig. 5b). The P(cation-adj-π) hydrogels were almost transparent, while the P(cation-co-π) hydrogels were opaque owing to the aggregation of their hydrophobic aromatic-rich segments. These hydrogels with different sequences also showed dramatically different mechanical strengths. In a 0.7 M NaCl solution, the P(cation-adj-π) hydrogels were soft but more stretchable and tough than the P(cation-co-π) hydrogels. The monomer sequence also had a strong effect on the underwater adhesion of these hydrogels (Fig. 5c). Adhesion tests showed that the P(cation-adj-π) hydrogels exhibited fast, strong, but reversible adhesion to negatively charged glass in artificial seawater because the aromatic groups enhanced the electrostatic interactions of their adjacent cationic residues with the counter surfaces in highly ionic media. In contrast, the P(cation-co-π) hydrogels exhibited weak adhesion on glass substrate in saltwater. This work indicated that the monomer sequence has a strong influence on the network structures and the properties of hydrogels, which has always been overlooked.
Soft materials with bioinspired microstructures are another type of tape-like adhesive. Although the corresponding works mainly focused on adhesion in air or water, instead of in saline water or seawater, it is expected that these materials have similar adhesion performances regardless of salinity due to their physical suction mechanism [29, 64,65,66,67]. This type of underwater adhesive has been widely reviewed in the literature and is not covered here [8, 23, 25, 27].
Conclusion and outlook
With an in-depth understanding of the underwater adhesion mechanisms of marine organisms, remarkable progress has been made in the development of adhesives applied in the marine environment. Especially in the last five years, various under-seawater adhesives have been fabricated by applying diverse bioinspired strategies [30, 32, 54]. However, compared with the development of adhesives used in the air, the development of adhesives that can be directly applied under-seawater is still in its infancy. Currently, the rapid formation of strong adhesion remains a major challenge. In real-world applications, the formation of adhesive joints occurs under nonstatic conditions, such as the constant undulation of seawater, which requires adhesives to form an effective adhesion in a short time. The currently reported glue-type adhesives exhibit strong bonding strength but require hours or even days of curing, while the tape-type adhesives show instant adhesion but relatively weak strength. Various length-scale adhesion mechanisms are used in nature to achieve excellent underwater adhesion. Therefore, further exploring the underwater adhesion mechanisms with various length scales of natural organisms and mimicking them is one direction for the development of under-seawater adhesives.
In addition, most adhesive joints used in marine environments are subjected to cyclic loadings throughout their life [68]. Therefore, the study of the long-term stability of adhesives under nonstatic conditions is essential for their real application in seawater. More comprehensive and systematic characterizations of the material properties of adhesives, such as fatigue, creep, and the effect of environmental changes (e.g., temperature, salinity, and hydraulic pressure), are required in future studies.
Moreover, to meet the requirements of diverse application scenarios, future under-seawater adhesives should also have controlled adhesion and multiple functionalities and be ecofriendly (recyclable, biodegradable, nontoxic, etc.), low cost, and easy to mass produce.
References
Legg M, Yücel MK, Garcia de Carellan I, Kappatos V, Selcuk C, Gan TH. Acoustic methods for biofouling control: A review. Ocean Eng. 2015;103:237–47.
Ting RY. A Study on Elastomer/Metal Bonds Applicable in Underwater Sonar Systems. In Adhesive Joints: Formation, Characteristics, and Testing, Mittal, KL Ed.; Springer US; Plenum Press, New York; 1984. pp 555–64.
Momber AW, Plagemann P, Stenzel V. The adhesion of corrosion protection coating systems for offshore wind power constructions after three years under offshore exposure. Int J Adhes Adhes. 2016;65:96–101.
Tserpes K, Barroso-Caro A, Carraro PA, Beber VC, Floros I, Gamon W, et al. A review on failure theories and simulation models for adhesive joints. J Adhes. 2022;98:1855–915.
Kinloch AJ. Interfacial Fracture Mechanical Aspects of Adhesive Bonded Joints—A Review. J Adhes. 1979;10:193–219.
Kerrison PD, Stanley MS, Hughes AD. Textile substrate seeding of Saccharina latissima sporophytes using a binder: An effective method for the aquaculture of kelp. Algal Res. 2018;33:352–7.
Zheng SY, Zhou J, Wang S, Wang Y-J, Liu S, Du G, et al. Water-Triggered Spontaneously Solidified Adhesive: From Instant and Strong Underwater Adhesion to In Situ Signal Transmission. Adv Funct Mater. 2022;32:2205597.
Fan HL, Gong JP. Bioinspired Underwater Adhesives. Adv Mater. 2021;33:2102983.
Narayanan A, Dhinojwala A, Joy A. Design principles for creating synthetic underwater adhesives. Chem Soc Rev. 2021;50:13321–45.
Cui C, Liu W. Recent advances in wet adhesives: Adhesion mechanism, design principle and applications. Prog Polym Sci. 2021;116:101388.
Israelachvili JN. Intermolecular and Surface Forces. 3rd ed. London: Elsevier; 2010.
Raviv U, Klein J. Fluidity of Bound Hydration Layers. Science. 2002;297:1540–3.
Maier GP, Rapp MV, Waite JH, Israelachvili JN, Butler A. Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science. 2015;349:628–32.
Wilker JJ. Positive charges and underwater adhesion. Science. 2015;349:582–3.
Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Mussel-Inspired Adhesives and Coatings. Annu Rev Mater Res. 2011;41:99–132.
Fan HL, Wang JH, Gong JP. Barnacle Cement Proteins-Inspired Tough Hydrogels with Robust, Long-Lasting, and Repeatable Underwater Adhesion. Adv Funct Mater. 2021;31:2009334.
Rao P, Sun TL, Chen L, Takahashi R, Shinohara G, Guo H, et al. Tough Hydrogels with Fast, Strong, and Reversible Underwater Adhesion Based on a Multiscale Design. Adv Mater. 2018;30:1801884.
Zhu X, Wei C, Chen H, Zhang C, Peng H, Wang D, et al. A Cation-Methylene-Phenyl Sequence Encodes Programmable Poly(Ionic Liquid) Coacervation and Robust Underwater Adhesion. Adv Funct Mater. 2021;32:2105464.
Ma X, Zhou X, Ding J, Huang B, Wang P, Zhao Y, et al. Hydrogels for underwater adhesion: adhesion mechanism, design strategies and applications. J Mater Chem A. 2022;10:11823–53.
Hofman AH, van Hees IA, Yang J, Kamperman M. Bioinspired Underwater Adhesives by Using the Supramolecular Toolbox. Adv Mater. 2018;30:1704640.
Ma S, Wu Y, Zhou F. Bioinspired synthetic wet adhesives: from permanent bonding to reversible regulation. Curr Opin Colloid Interface Sci. 2020;47:84–98.
Cui M, Ren S, Wei S, Sun C, Zhong C. Natural and bio-inspired underwater adhesives: Current progress and new perspectives. APL Mater. 2017;5:116102.
Chen Y, Meng J, Gu Z, Wan X, Jiang L, Wang S. Bioinspired Multiscale Wet Adhesive Surfaces: Structures and Controlled Adhesion. Adv Funct Mater. 2019;30:1905287.
Xu L, Huang Z, Deng Z, Du Z, Sun TL, Guo Z-H, et al. A Transparent, Highly Stretchable, Solvent-Resistant, Recyclable Multifunctional Ionogel with Underwater Self-Healing and Adhesion for Reliable Strain Sensors. Adv Mater. 2021;33:2105306.
Liu X, Yu H, Wang L, Huang Z, Haq F, Teng L, et al. Recent Advances on Designs and Applications of Hydrogel Adhesives. Adv Mater Interfaces. 2022;9:2101038.
Wang S, Liu J, Wang L, Cai H, Wang Q, Wang W, et al. Underwater Adhesion and Anti-Swelling Hydrogels. Adv Mater Technol. 2022;n/a:2201477. https://doi.org/10.1002/admt.202201477.
Yin Yuen H, Ho Pan Bei B, Zhao X. Underwater and wet adhesion strategies for hydrogels in biomedical applications. Chem Eng J. 2021;431:133372.
Nam S, Mooney D. Polymeric Tissue Adhesives. Chem Rev. 2021;121:11336–84.
Baik S, Kim DW, Park Y, Lee T-J, Ho Bhang S, Pang C. A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature. 2017;546:396.
Fan HL, Wang JH, Tao Z, Huang JC, Rao P, Kurokawa T, et al. Adjacent cationic–aromatic sequences yield strong electrostatic adhesion of hydrogels in seawater. Nat Commun. 2019;10:5127.
Akdogan Y, Wei W, Huang K-Y, Kageyama Y, Danner EW, Miller DR, et al. Intrinsic Surface-Drying Properties of Bioadhesive Proteins. Angew Chem Int Ed. 2014;53:11253–6.
White JD, Wilker JJ. Underwater Bonding with Charged Polymer Mimics of Marine Mussel Adhesive Proteins. Macromolecules. 2011;44:5085–8.
Yuk H, Varela CE, Nabzdyk CS, Mao X, Padera RF, Roche ET, et al. Dry double-sided tape for adhesion of wet tissues and devices. Nature. 2019;575:169–74.
Beharaj A, McCaslin EZ, Blessing WA, Grinstaff MW. Sustainable polycarbonate adhesives for dry and aqueous conditions with thermoresponsive properties. Nat Commun. 2019;10:5478.
Su X, Luo Y, Tian Z, Yuan Z, Han Y, Dong R, et al. Ctenophore-inspired hydrogels for efficient and repeatable underwater specific adhesion to biotic surfaces. Mater Horiz. 2020;7:2651–61.
Li X, Deng Y, Lai J, Zhao G, Dong S. Tough, Long-Term, Water-Resistant, and Underwater Adhesion of Low-Molecular-Weight Supramolecular Adhesives. J Am Chem Soc. 2020;142:5371–9.
Fan HL, Wang JH, Jin ZX. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-Cross-Linked Hydrogels Based on Polymer–Tannic Acid Multiple Hydrogen Bonds. Macromolecules 2018;51:1696–705.
Gong JP. Materials both Tough and Soft. Science. 2014;344:161–2.
Cui K, Gong JP. Aggregated structures and their functionalities in hydrogels. Aggregate. 2021;2:e33.
Ahn BK. Perspectives on Mussel-Inspired Wet Adhesion. J Am Chem Soc. 2017;139:10166–71.
Liu Y, Ai K, Lu L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem Rev. 2014;114:5057–115.
Jin ZX, Fan HL. The modulation of melanin-like materials: methods, characterization and applications. Polym Int. 2016;65:1258–66.
Guo Q, Chen J, Wang J, Zeng H, Yu J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale. 2020;12:1307–24.
Fan HL, Wang JH, Zhang QY, Jin ZX. Tannic Acid-Based Multifunctional Hydrogels with Facile Adjustable Adhesion and Cohesion Contributed by Polyphenol Supramolecular Chemistry. ACS Omega. 2017;2:6668–76.
North MA, Del Grosso CA, Wilker JJ. High Strength Underwater Bonding with Polymer Mimics of Mussel Adhesive Proteins. ACS Appl Mater Interfaces. 2017;9:7866–72.
Zhan K, Kim C, Sung K, Ejima H, Yoshie N. Tunicate-Inspired Gallol Polymers for Underwater Adhesive: A Comparative Study of Catechol and Gallol. Biomacromolecules 2017;18:2959–66.
Sha X, Zhang C, Qi M, Zheng L, Cai B, Chen F, et al. Mussel-Inspired Alternating Copolymer as a High-Performance Adhesive Material Both at Dry and Under-Seawater Conditions. Macromol Rapid Commun. 2020;41:2000055.
Li A, Mu Y, Jiang W, Wan X. A mussel-inspired adhesive with stronger bonding strength under underwater conditions than under dry conditions. Chem Commun. 2015;51:9117–20.
Mu Y, Wu X, Pei D, Wu Z, Zhang C, Zhou D, et al. Contribution of the Polarity of Mussel-Inspired Adhesives in the Realization of Strong Underwater Bonding. ACS Biomater Sci Eng. 2017;3:3133–40.
Kim S, Yoo HY, Huang J, Lee Y, Park S, Park Y, et al. Salt Triggers the Simple Coacervation of an Underwater Adhesive When Cations Meet Aromatic π Electrons in Seawater. ACS Nano. 2017;11:6764–72.
Cui C, Gu R, Wu T, Yuan Z, Fan C, Yao Y, et al. Zwitterion-Initiated Spontaneously Polymerized Super Adhesive Showing Real-Time Deployable and Long-Term High-Strength Adhesion against Various Harsh Environments. Adv Funct Mater. 2022;32:2109144.
Peng Q, Wu Q, Chen J, Wang T, Wu M, Yang D, et al. Coacervate-Based Instant and Repeatable Underwater Adhesive with Anticancer and Antibacterial Properties. ACS Appl Mater Interfaces. 2021;13:48239–51.
Niu W, Zhu J, Zhang W, Liu X. Simply Formulated Dry Pressure-Sensitive Adhesives for Substrate-Independent Underwater Adhesion. ACS Mater Lett. 2022;4:410–7.
Yu Z, Wu P. Underwater Communication and Optical Camouflage Ionogels. Adv Mater. 2021;33:2008479.
Han L, Wang M, Prieto-López LO, Deng X, Cui J. Self-Hydrophobization in a Dynamic Hydrogel for Creating Nonspecific Repeatable Underwater Adhesion. Adv Funct Mater. 2020;30:1907064.
Liu X, Zhang Q, Duan L, Gao G. Tough Adhesion of Nucleobase-Tackifed Gels in Diverse Solvents. Adv Funct Mater. 2019;29:1900450.
Liu X, Zhang Q, Duan L, Gao G. Bioinspired Nucleobase-Driven Nonswellable Adhesive and Tough Gel with Excellent Underwater Adhesion. ACS Appl Mater Interfaces. 2019;11:6644–51.
Zhang Z, Guo L, Hao J. Emulsion-Based Organohydrogels with Switchable Wettability and Underwater Adhesion toward Durable and Ecofriendly Marine Antifouling Coatings. ACS Appl Polym Mater. 2021;3:3060–70.
Waite JH. Mussel adhesion—essential footwork. J Exp Biol. 2017;220:517–30.
Rocha M, Antas P, Castro LFC, Campos A, Vasconcelos V, Pereira F, et al. Comparative Analysis of the Adhesive Proteins of the Adult Stalked Goose Barnacle Pollicipes pollicipes (Cirripedia: Pedunculata). Mar Biotechnol. 2018;21:38–51.
Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, et al. Pre-fusion structure of a human coronavirus spike protein. Nature 2016;531:118–21.
DeStefano AJ, Segalman RA, Davidson EC. Where Biology and Traditional Polymers Meet: The Potential of Associating Sequence-Defined Polymers for Materials Science. JACS Au. 2021;1:1556–71.
Fan HL, Cai YR, Gong JP. Facile Tuning of Hydrogel Properties by Manipulating Cationic-Aromatic Monomer Sequences. Sci China Chem. 2021;64:1560–8.
Baik S, Kim J, Lee HJ, Lee TH, Pang C. Highly Adaptable and Biocompatible Octopus-Like Adhesive Patches with Meniscus-Controlled Unfoldable 3D Microtips for Underwater Surface and Hairy Skin. Adv Sci. 2018;5:1800100.
Wang Y, Kang V, Arzt E, Federle W, Hensel R. Strong Wet and Dry Adhesion by Cupped Microstructures. ACS Appl Mater Interfaces. 2019;11:26483–90.
Zheng H, Li J, Zhou Y, Zhang C, Xu W, Deng Y, et al. Electrically switched underwater capillary adhesion. Nat Commun. 2022;13:4584.
Frey ST, Haque ABMT, Tutika R, Krotz EV, Lee C, Haverkamp CB, et al. Octopus-inspired adhesive skins for intelligent and rapidly switchable underwater adhesion. Sci Adv. 2022;8:eabq1905.
Costa M, Viana G, da Silva LFM, Campilho RDSG. Environmental effect on the fatigue degradation of adhesive joints: A review. J Adhes. 2017;93:127–46.
Dickson AG, Goyet C. Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water. Version 2. United States; ORNL/CDIAC-74, Carbon Dioxide Inf. and Anal. Cent., Oak Ridge, Natl. Lab., Oak Ridge, Tenn; 1994. Available at http://cdiac.ornl.gov/oceans/handbook.html.
Yan Y, Huang J, Qiu X, Zhuang D, Liu H, Huang C, et al. A Strong Underwater Adhesive that Totally Cured in Water. Chem Eng J. 2021;431:133460.
Li Y, Huang X, Xu Y, Ma C, Cai L, Zhang J, et al. A bio-inspired multifunctional soy protein-based material: From strong underwater adhesion to 3D printing. Chem Eng J. 2021;430:133017.
Das S, Vasilyev G, Martin P, Zussman E. Bioinspired Cationic-Aromatic Copolymer for Strong and Reversible Underwater Adhesion. ACS Appl Mater Interfaces. 2022;14:26287–94.
Cai C, Zhu H, Chen Y, Chen C, Li H, Yang Z, et al. Conductive nerve guide conduits based on wet-adhesive hydrogel to accelerate peripheral nerve repair. Appl Mater Today. 2022;27:101491.
Sun C, Luo J, Jia T, Hou C, Li Y, Zhang Q, et al. Water-resistant and underwater adhesive ion-conducting gel for motion-robust bioelectric monitoring. Chem Eng J. 2022;431:134012.
Acknowledgements
This research was supported by JSPS KAKENHI (Grant Numbers JP21K14676) and the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) established by the World Premier International Research Initiative (WPI), MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, H. Getting glued in the sea. Polym J 55, 653–664 (2023). https://doi.org/10.1038/s41428-023-00769-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00769-6
This article is cited by
-
Gallol-Containing Polymers: Synthesis and Applications
Chemistry Africa (2023)