Abstract
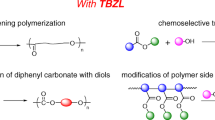
Benzyl triflate (BnOTf) was demonstrated as a new initiator in cationic ring-opening polymerization (ROP) of cyclic dithiocarbonate, yielding poly(dithiocarbonate) with a benzylated chain end. BnOTf was generated in situ from benzyl bromide and silver trifluoromethanesulfonate (AgOTf). Several arylmethyl triflates were prepared from 1,4-bis(bromomethyl)benzene, 1,3,5-tris(bromomethyl)benzene, and 1,2,4,5-tetrakis(bromomethyl)benzene. These triflates were used for the synthesis of polymeric architectures, such as two-armed linear polymers and tri- and tetra-armed polymers. The number of polymer arms was related to the number of arylmethyl triflate groups bound in the initiators through divergent chain propagation. The chain length of each arm was adjusted with initiators at theoretical concentrations considering the number of substituted triflates and confirmed via 1H NMR analysis of the prepared polymers. The lengths agreed with the results determined by the number-average molecular weights retrieved from gel permeation chromatography analysis. A symmetric structure of the aromatic cores and low polydispersities of the prepared polymers were suggested for divergent chain propagation. Fluorescent dye-attached polymers were prepared through ROP, and the presence of cores retrieved from the initiators was verified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cowie JMG, Valeria A. Polymers chemistry and physics of modern materials. 3rd edn., Boca Raton: Taylor & Francis, 2007.
Grubbs RB, Grubbs RH. 50th anniversary perspective: living polymerization-emphasizing the molecule in macromolecules. Macromolecules. 2017;50:6979–97.
Bingham MN, Abousalman-Rezvani Z, Collins K, Roth PJ. Thiocarbonyl chemistry in polymer science. Polym Chem 2022;13:2880–01.
Dey A, Haldar U. Block PDe. Copolymer synthesis by the combination of living cationic polymerization and other polymerization methods. Front Chem 2021;9:644–47.
Darensbourg DJ, Andreatta JR, Jungman MJ, Reibenspies JH. Investigation into the coupling of cyclohexene oxide and carbon isulfide catalyzed by (salen)CrCl. Selectivity for the production of copolymers vs. cyclic thiocarbonates. Dalton Trans. 2009; 8891–99.
Tang Y, Pina-Hernandez C, Niu Q, Nie J, Cabrini S. A novel high-refractive index episulfide-thiol polymer for nanoimprinting optical elements. J Mater Chem C. 2018;6:8823–31.
Nakano K, Tatsumi G, Nozaki K. Synthesis of sulfur-rich polymers: copolymerization of episulfide with carbon disulfide by using [PPN]Cl/salph)Cr(III)Cl system. J Am Chem Soc. 2007;129:15116–17.
Nuyken O, Pask SD. Ring-opening polymerization-an introductory review. Polym. 2013;5:361–403.
Gleede T, Reisman L, Rieger E, Mbarushiman PC, Rupar PA, Wurm FR. Aziridines and azetidines: building blocks for polyamines by anionic and cationic ring-opening polymerization. Polym Chem 2019;10:3257–83.
Krishnamurthy S, Yoshida Y, Endo T. Cationic ring-opening polymerization of a five membered cyclic dithiocarbonate having a tertiary amine moiety. Polym Chem 2022;13:267–74.
Do JY, Shin SB, Jeong SM, Jung M. Ring-opening polymerization of cyclic 1,3-oxathiolane-2-thione promoted by neighboring sulfide group and ring contraction. Eur Polym J 2020;131:109689.
Choi W, Sanda F, Endo T. Dependence of ring-opening reaction of five-membered dithiocarbonates on cationic catalyst: control of isomerization and polymerization. Macromolecules 1998;31:2454–60.
Kim CG, Son MJ, Do JY. Cationic living polymerization of cyclic dithiocarbonates involving sulfide-migration. Eur Polym J 2021;156:110611.
Oike H, Imaizumi H, Mouri T, Yoshioka Y, Uchibori A. Designing unusual polymer topologies by electrostatic self-assembly and covalent fixation. J Am Chem Soc. 2000;122:9592–99.
Laali K. Electrophilic benzylation and nitration of 2,6-dimethylanisole, 2,6-dimethylphenol, and 2,6-diisopropylphenol. Isomer distribution and mechanistic considerations. J Org Chem. 1985;50:3638–40.
Allison-Logan S, Karimi F, Sun Y, McKenzie TG, Nothling MD, Bryant G, et al. Highly living stars via core-first photo-RAFT polymerization: exploitation for ultra-high molecular weight star synthesis. ACS Macro Lett. 2019;8:1291–95.
Yang DP, Oo MNNL, Deen GR, Li Z, Loh XJ. Nano-star-shaped polymers for drug delivery applications. Macromol Rapid Commun 2017;38:1700410.
Hasneen A, Kim SJ, Paik H. Synthesis and characterization of low molecular weight poly(methyl acrylate)-b-polystyrene by a combination of ATRP and click coupling method. Macromol Res 2007;15:541–46.
Chesnokov GA, Topchiy MA, Dzhevakov PB, Gribanov PS, Tukov AA, Khrustalev VN, et al. Eight-membered-ring diaminocarbenes bearing naphthalene moiety in the backbone: DFT studies, synthesis of amidinium salts, generation of free carbene, metal complexes, and solvent-free copper catalyzed azide-alkyne cycloaddition (CuAAC) reaction. Dalton Trans. 2017;46:4331–45.
Callaghan S, Flanagan KJ, O’Brien JE, Senge MO. Short-chained anthracene strapped porphyrins and their endoperoxides. Eur J Org Chem. 2020;2020:2735–44.
Nallasivam JL, Fernandes RA. A cascade aza-cope/aza-prins cyclization leading to piperidine derivatives. Eur J Org Chem. 2015;2015:2012–22.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2019R1F1A1060583).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, C.G., Jeong, H.J. & Do, J.Y. Divergent chain growth of poly(dithiocarbonate)s through arylmethyl triflate-mediated ring-opening polymerization of cyclic dithiocarbonate. Polym J 55, 571–580 (2023). https://doi.org/10.1038/s41428-022-00745-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00745-6