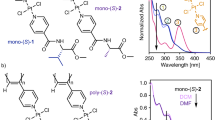
Abstract
Poly(3-alkylesterfurans) fold into helical conformations due to a preference for adjacent furan repeat units in the polymer backbone to adopt a syn conformation in which the oxygen atoms of the furan rings are oriented in the same direction. In this contribution, a new chiral helical poly(3-alkylesterfuran) is reported in which a cholesterol group has been attached to the backbone to ensure excess single-handed helix chirality. This rigid side group promotes a folded conformation of the polymer in chloroform, as evidenced by 1H NMR, absorption, and circular dichroism studies. This is unique among previously reported helical poly(3-alkylesterfurans) with S-3-octanol side chains, as those polymers are much more disordered in the same solvent. Circularly polarized luminescence studies also indicated how the chiroptical properties are impacted by intermolecular aggregation of these helical polymers. The study reveals the potential for tuning the properties of helical polyfurans via precise choice of the chiral side chain, which can be installed during monomer synthesis via a Steglich modification of 2-bromo-3-furoic acid. Suzuki-Miyaura cross-coupling polymerization produces the desired chiral helical polymers in reasonable yields with molecular weights above 10 kg/mol when the commercially available PEPPSI-IPent catalyst is used to catalyze cross-coupling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yashima E, Ousaka N, Taura D, Shimomura K, Ikai T, Maeda K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem Rev. 2016;116:13752–990.
Yashima E, Maeda K, Iida H, Furusho Y, Nagai K. Helical Polymers: Synthesis, Structures, and Functions. Chem Rev. 2009;109:6102–211.
Nakano T, Okamoto Y. Synthetic Helical Polymers: Conformation and Function. Chem Rev. 2001;101:4013–38.
Verswyvel M, Koeckelberghs G. Chirality in Conjugated Polymers: When Two Components Meet. Polym Chem. 2012;3:3203–16.
Kane-Maguire LAP, Wallace GG. Chiral Conducting Polymers. Chem Soc Rev. 2010;39:2545–76.
Freire F, Quiñoá E, Riguera R. Supramolecular Assemblies from Poly(phenylacetylene)s. Chem Rev. 2016;116:1242–71.
Brunsveld L, Prince RB, Meijer EW, Moore JS. Conformational Ordering of Apolar, Chiral m-Phenylene Ethynylene Oligomers. Org Lett. 2001;2:1525–8.
Brunsveld L, Meijer EW, Prince RB, Moore JS. Self-Assembly of Folded m-Phenylene Ethynylene Oligomers into Helical Columns. J Am Chem Soc. 2001;123:7978–84.
Hu Y, Song F, Xu Z, Tu Y, Zhang H, Cheng Q, et al. Circularly Polarized Luminescence from Chiral Conjugated Poly(carbazole-ran-acridine)s with Aggregation-Induced Emission and Delayed Fluorescence. ACS Appl Polym Mater. 2019;1:221–9.
Vanormelingen W, Smeets A, Franz E, Asselberghs I, Clays K, Verbiest T, et al. Conformational Steering in Substituted Poly(3,6-phenanthrene)s: A Linear and Nonlinear Optical Study. Macromolecules 2009;42:4282–7.
Vanormelingen W, Van den Bergh K, Verbiest T, Koeckelberghs G. Conformational Transitions in Chiral, Gallic Acid-Functionalized Poly(dithienopyrrole): A Comparative UV-vis and CD Study. Macromolecules 2008;41:5582–9.
Wang Y, Harada T, Phuong LQ, Kanemitsu Y, Nakano T. Helix Induction to Polyfluorenes Using Circularly Polarized Light: Chirality Amplification, Phase-Selective Induction, and Anisotropic Emission. Macromolecules 2018;51:6865–77.
Wang K, Xiao Y. Chirality in Polythiophenes: A Review. Chirality 2021;33:424–46.
Ewbank PC, Stefan MC, Sauvé G, McCullough RD. Synthesis, Characterization and Properties of Regioregular Polythiophene-Based Materials. In Handbook of Thiophene-Based Materials. In: Perepichka, IF;, Perepichka DF, editors. Chichester, UK: John Wiley & Sons, Ltd; 2009. p. 157–217.
McCullough RD, Lowe RD. Enhanced Electrical Conductivity in Regioselectively Synthesized Poly(3-alkylthiophenes). J Chem Soc Chem Commun. 1992;70–2.
Miyakoshi R, Yokoyama A, Yokozawa T. Synthesis of Poly(3-hexylthiophene) with a Narrower Polydispersity. Macromol Rapid Commun. 2004;25:1663–6.
Sheina EE, Liu J, Iovu MC, Laird DW, McCullough RD. Chain Growth Mechanism for Regioregular Nickel-Initiated Cross-Coupling Polymerizations. Macromolecules 2004;37:3526–8.
Yokoyama A, Miyakoshi R, Yokozawa T. Chain-Growth Polymerization for Poly(3-hexylthiophene) with a Defined Molecular Weight and a Low Polydispersity. Macromolecules 2004;37:1169–71.
Leysen P, Teyssandier J, De Feyter S, Koeckelberghs G. Controlled Synthesis of a Helical Conjugated Polythiophene. Macromolecules 2018;51:3504–14.
Wang P, Jeon I, Lin Z, Peeks MD, Savagatrup S, Kooi SE, et al. Insights into Magneto-Optics of Helical Conjugated Polymers. J Am Chem Soc. 2018;140:6501–8.
Ikai T, Takayama K, Wada Y, Minami S, Apiboon C, Shinohara K. Synthesis of a One-Handed Helical Polythiophene: A New Approach Using an Axially Chiral Bithiophene with a Fixed syn-conformation. Chem Sci. 2019;10:4890–5.
Gidron O, Dadvand A, Wei-Hsin Sun E, Chung I, Shimon LJW, Bendikov M, et al. Oligofuran-Containing Molecules for Organic Electronics. J Mater Chem C. 2013;1:4358–67.
Gidron O, Bendikov M. α-Oligofurans: An Emerging Class of Conjugated Oligomers for Organic Electronics. Angew Chem Int Ed. 2014;53:2546–55.
Jin X-H, Sheberla D, Shimon LJW, Bendikov M. Highly Coplanar Very Long Oligo(alkylfuran)s: A Conjugated System with Specific Head-To-Head Defect. J Am Chem Soc. 2014;136:2592–601.
Varni AJ, Fortney A, Baker MA, Worch JC, Qiu Y, Yaron D, et al. Photostable Helical Polyfurans. J Am Chem Soc. 2019;141:8858–67.
Jones GIL, Owen NL. Molecular Structure and Conformation of Carboxylic Esters. J Mol Struct. 1973;18:1–32.
Bloom JWG, Wheeler SE. Benchmark Torsional Potentials of Building Blocks for Conjugated Materials: Bifuran, Bithiophene, and Biselenophene. J Chem Theory Comput. 2014;10:3647–55.
Lin JB, Jin Y, Lopez SA, Druckerman N, Wheeler SE, Houk KN. Torsional Barriers to Rotation and Planarization in Heterocyclic Oligomers of Value in Organic Electronics. J Chem Theory Comput. 2017;13:5624–38.
Topolskaia V, Pollit AA, Cheng S, Seferos DS. Trends in Conjugated Chalcogenophenes: A Theoretical Study. Chem Eur J. 2021;27:9038–43.
Chotana GA, Kallepalli VA, Maleczka RE, Smith MR. Iridium-Catalyzed Borylation of Thiophenes: Versatile, Synthetic Elaboration Founded on Selective C-H Functionalization. Tetrahedron 2008;64:6103–14.
Valente C, Çalimsiz S, Hoi KH, Mallik D, Sayah M, Organ MG. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew Chem Int Ed. 2012;51:3314–32.
Kawakami M, Schulz KHG, Varni AJ, Tormena CF, Gil RR, Noonan KJT. Statistical Copolymers of Thiophene-3-carboxylates and Selenophene-3-carboxylates; 77Se NMR as a Tool to Examine Copolymer Sequence in Selenophene-based Conjugated Polymers. Polym Chem. 2022;13:5316–24.
Duong ST, Fujiki M. The Origin of Bisignate Circularly Polarized Luminescence (CPL) Spectra from Chiral Polymer Aggregates and Molecular Camphor: anti-Kasha’s Rule Revealed by CPL Excitation (CPLE) Spectra. Polym Chem. 2017;8:4673–9.
Acknowledgements
KJTN and SB are grateful to the NSF for support of this work (CHE-2109065 and CHE-2102460).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawakami, M., Downey, P., Peteanu, L. et al. Cholesterol side groups in Helical Poly(3-alkylesterfurans). Polym J 55, 565–570 (2023). https://doi.org/10.1038/s41428-022-00741-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00741-w