Abstract
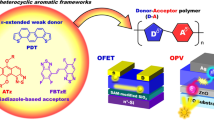
Fused π-conjugated molecules have been extensively developed as critical and indispensable components in organic semiconductors due to their desirable physicochemical properties and charge-transport characteristics. Incorporation of acceptor units into fused π-conjugated frameworks has advantages in terms of tuning frontier molecular orbital energies. However, development of fused π-conjugated molecules with donor-acceptor configurations is still lacking due to limited availability and synthetic access to acceptor units. Here, we report the design and synthesis of a new fused donor-acceptor-donor π-conjugated molecule (BDT-NTz) composed of electron-donating benzodithiophene (BDT) units and an electron-accepting naphthobisthiadiazole (NTz) unit. The fused chemical structure of BDT-NTz generated a sharp absorption band and a high quantum efficiency. This indicated that BDT-NTz showed typical p-type responses with a hole mobility of 1.1 × 10−3 cm2 V−1 s−1 in an organic field-effect transistor. We also developed a copolymer based on BDT-NTz and an electron-accepting fluorinated NTz and investigated its photophysical/electrochemical properties and semiconducting characteristics. This study demonstrates that the use of fused π-extended molecules containing NTz can broaden the availability of both small-molecule-based and polymer-based organic semiconducting materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Padinger F, Rittberger RS, Sariciftci NS. Effects of postproduction treatment on plastic solar cells. Adv Funct Mater. 2003;13:85–88.
Takimiya K, Ebata H, Sakamoto K, Izawa T, Otsubo T, Kunugi Y. 2,7-Diphenyl[1]benzothieno[3,2-b]benzothiophene, a new organic semiconductor for air-stable organic field-effect transistors with mobilities up to 2.0 cm2 V−1 s−1. J Am Chem Soc. 2006;128:12604–5.
Pisula W, Feng X, Müllen K. Charge-carrier transporting graphene-type molecules. Chem Mater. 2011;23:554–67.
Zhang H, Zhang S, Gao K, Liu F, Yao H, Yang B, et al. Low band-gap conjugated polymer based on diketopyrrolopyrrole units and its application in organic photovoltaic cells. J Mater Chem A. 2017;5:10416–23.
Zhang L, Cao Y, Colella NS, Liang Y, Brédas JL, Houk KN, et al. Unconventional, chemically stable, and soluble two-dimensional angular polycyclic aromatic hydrocarbons: from molecular design to device applications. Acc Chem Res. 2015;48:500–9.
Umeyama T, Igarashi K, Sasada D, Tamai Y, Ishida K, Koganezawa T, et al. Efficient light-harvesting, energy migration, and charge transfer by nanographene-based nonfullerene small-molecule acceptors exhibiting unusually long excited-state lifetime in the film state. Chem Sci. 2020;11:3250–7.
Takimiya K, Bulgarevich K, Abbas M, Horiuchi S, Ogaki T, Kawabata K, et al. “Manipulation” of crystal structure by methylthiolation enabling ultrahigh mobility in a pyrene-based molecular semiconductor. Adv Mater. 2021;33:2102914.
Yu CP, Kumagai S, Kushida T, Mitani M, Mitsui C, Ishii H, et al. Mixed-orbital charge transport in n-shaped benzene- and pyrazine-fused organic semiconductors. J Am Chem Soc. 2022;144:11159–11167.
Kitamura M, Arakawa Y. Pentacene-based organic field-effect transistors. J Phys Condens Matter. 2008;20:184011.
Zhan X, Facchetti A, Barlow S, Marks TJ, Ratner MA, Wasielewski MR, et al. Rylene and related diimides for organic electronics. Adv Mater. 2011;23:268–84.
Markiewicz JT, Wudl F. Perylene, oligorylenes, and aza-analogs. ACS Appl Mater Interfaces. 2015;7:28063–28085.
Cui X, Xiao C, Winands T, Koch T, Li Y, Zhang L, et al. Hexacene diimides. J Am Chem Soc. 2018;140:12175–80.
Lin Y, He Q, Zhao F, Huo L, Mai J, Lu X, et al. A facile planar fused-ring electron acceptor for as-cast polymer solar cells with 8.71% efficiency. J Am Chem Soc. 2016;138:2973–6.
Cui Y, Yao H, Zhang J, Zhang T, Wang Y, Hong L, et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat Commun. 2019;10:2515.
Yuan J, Zhang Y, Zhou L, Zhang C, Lau TK, Zhang G, et al. Fused benzothiadiazole: a building block for n-type organic acceptor to achieve high-performance organic solar cells. Adv Mater. 2019;31:1807577.
Lai H, Chen H, Zhu Y, Chen L, Huang HH, He F. An asymmetrical A-DAD-A-type acceptor simultaneously enhances voltage and current for efficient organic solar cells. J Mater Chem A. 2020;8:9670–6.
Facchetti A, Deng Y, Wang A, Koide Y, Sirringhaus H, Marks TJ, et al. Tuning the semiconducting properties of sexithiophene by α,ω-substitution- α,ω-diperfluorohexylsexithiophene: the first n-type sexithiophene for thin-film transistors. Angew Chem Int Ed. 2000;39:4547–4551.
Sakamoto Y, Komatsu S, Suzuki T. Tetradecafluorosexithiophene: the first perfluorinated oligothiophene. J Am Chem Soc. 2001;123:4643–4.
Sakamoto Y, Suzuki T, Kobayashi M, Gao Y, Fukai Y, Inoue Y, et al. Perfluoropentacene: high-performance p-n junctions and complementary circuits with pentacene. J Am Chem Soc. 2004;126:8138–40.
Ie Y, Nitani M, Ishikawa M, Nakayama K, Tada H, Kaneda T, et al. Electronegative oligothiophenes for n-type semiconductors: difluoromethylene-bridged bithiophene and its oligomers. Org Lett. 2007;9:2115–8.
Park SK, Kim JH, Yoon SJ, Kwon OK, An BK, Park SY. High-performance n-type organic transistor with a solution-processed and exfoliation-transferred two-dimensional crystalline layered film. Chem Mater. 2012;24:3263–8.
Ie Y, Jinnai S, Nitani M, Aso Y. Arenedithiocarboxyimide-containing extended π-conjugated systems with high electron affinity. J Mater Chem C. 2013;1:5373–80.
Melucci M, Durso M, Bettini C, Gazzano M, Maini L, Toffanin S, et al. Structure-property relationships in multifunctional thieno(bis)imide-based semiconductors with different sized and shaped n-alkyl ends. J Mater Chem C. 2014;2:3448–56.
Cheng YJ, Chen CH, Ho YJ, Chang SW, Witek HA, Hsu CS. Thieno[3,2-b]pyrrolo donor fused with benzothiadiazolo, benzoselenadiazolo and quinoxalino acceptors: synthesis, characterization, and molecular properties. Org Lett. 2011;13:5484–7.
Yang Y, Wang Y, Xie Y, Xiong T, Yuan Z, Zhang Y, et al. Fused perylenebisimide-carbazole: new ladder chromophores with enhanced third-order nonlinear optical activities. Chem Commun. 2011;47:10749–51.
Kojima M, Tamoto A, Aratani N, Yamada H. Rearrangement of an aniline linked perylene bisimide under acidic conditions and visible to near-infrared emission from the intramolecular charge-transfer state of its fused derivatives. Chem Commun. 2017;53:5698–701.
Cai Z, Zhang N, Awais MA, Filatov AS, Yu L. Synthesis of alternating donor–acceptor ladder‐type molecules and investigation of their multiple charge‐transfer pathways. Angew Chem Int Ed. 2018;57:6442–8.
Zhou Z, Liu W, Zhou G, Zhang M, Qian D, Zhang J, et al. Subtle molecular tailoring induces significant morphology optimization enabling over 16% efficiency organic solar cells with efficient charge generation. Adv Mater. 2020;32:1906324.
Su F, Chen S, Mo X, Wu K, Wu J, Lin W, et al. Trisulfur radical anion-triggered stitching thienannulation: rapid access to largely π-extended thienoacenes. Chem Sci. 2020;11:1503–9.
Wen J, Qiu F, Liu H, Liu X, Hu H, Duan Y, et al. syn/anti-oligothienoacene diimides with up to 10 fused rings. Angew Chem Int Ed. 2022;61:e202112482.
Wang M, Hu X, Liu P, Li W, Gong X, Huang F, et al. Donor-acceptor conjugated polymer based on naphtho[1,2-c:5,6-c’]bis[1,2,5]thiadiazole for high-performance polymer solar cells. J Am Chem Soc. 2011;133:9638–41.
Osaka I, Takimiya K. Naphthobischalcogenadiazole conjugated polymers: emerging materials for organic electronics. Adv Mater. 2017;29:1605218.
Chatterjee S, Ie Y, Karakawa M, Aso Y. Naphtho[1,2‐c:5,6‐c’]bis[1,2,5]thiadiazole‐containing π‐conjugated compound: nonfullerene electron acceptor for organic photovoltaics. Adv Funct Mater. 2016;26:1161–1168.
Chatterjee S, Ie Y, Seo T, Moriyama T, Wetzelaer GJAH, Blom PWM, et al. Fluorinated naphtho[1,2-c:5,6-c’]bis[1,2,5]thiadiazole-containing π-conjugated compound: synthesis, properties, and acceptor applications in organic solar cells. NPG Asia Mater. 2018;10:1016–28.
Iguchi K, Mikie T, Saito M, Komeyama K, Seo T, Ie Y, et al. N-type semiconducting polymers based on dicyano naphthobisthiadiazole: high electron mobility with unfavorable backbone twist. Chem Mater. 2021;33:2218–28.
Jinnai S, Oi A, Seo T, Moriyama T, Minami R, Higashida S, et al. Electron-accepting π-conjugated compound containing cyano-substituted naphthobisthiadiazole as nonfullerene acceptor in organic solar cells. Synthesis. 2021;53:3390–6.
Aldrich TJ, Dudnik AS, Eastham ND, Manley EF, Chen LX, Chang RPH, et al. Suppressing defect formation pathways in the direct C-H arylation polymerization of photovoltaic copolymers. Macromolecules. 2018;51:9140–55.
Nielsen CB, White AJP, McCulloch I. Effect of fluorination of 2,1,3-benzothiadiazole. J Org Chem. 2015;80:5045–8.
Conboy G, Spencer HJ, Angioni E, Kanibolotsky AL, Findlay NJ, Coles SJ, et al. To bend or not to bend-are heteroatom interactions within conjugated molecules effective in dictating conformation and planarity? Mater Horiz. 2016;3:333–9.
Thorley KJ, McCulloch I. Why are S-F and S-O non-covalent interactions stabilising? J Mater Chem C. 2018;6:12413–21.
da Silva Filho DA, Kim E-G, Brédas J-L. Transport Properties in the Rubrene Crystal: Electronic Coupling and Vibrational Reorganization Energy. Adv Mater. 2005;17:1072–5.
Turro NJ, Ramamurthy V, Scaiano JC. Principles of molecular photochemistry: an introduction. Sausalito, CA: University Science Books; 2009.
Yoshida H. Near-ultraviolet inverse photoemission spectroscopy using ultra-low energy electrons. Chem Phys Lett. 2012;539–540:180–5.
Yoshida H. Measuring the electron affinity of organic solids: an indispensable new tool for organic electronics. Anal Bioanal Chem. 2014;406:2231–7.
Yoshida H. Electron transport in bathocuproine interlayer in organic semiconductor devices. J Phys Chem C. 2015;119:24459–64.
Jinnai S, Ie Y, Kashimoto H, Yoshida H, Karakawa M, Aso Y. Three-dimensional π-conjugated compounds as non-fullerene acceptors in organic photovoltaics: the influence of acceptor unit orientation at phase interfaces on photocurrent generation efficiency. J Mater Chem A. 2017;5:3932–8.
Acknowledgements
This work was supported by JSPS KAKENHI (20H02814, 20H05841, 20KK0123, 19K15505, 20H04639, and 21K14602), CREST (J205101030), NEDO (21500248-0), and “Dynamic Alliance for Open Innovation Bridging Human, Environmental and Materials” from the Ministry of Education, Culture, Sports, Science and Technology, Japan. YI is grateful to the Takahashi Industrial and Economic Research Foundation and NAGASE Science Technology Foundation. Thanks are extended to the CAC, ISIR, for assistance in obtaining elemental analyses. We appreciate Prof. Dr. Kenji Matsuda (Kyoto University) and Dr. Daiki Shimizu (Kyoto University) for the measurement of fluorescence lifetime and absolute quantum yield. We also wish to express our appreciation to Prof. Dr. Akinori Saeki (Osaka University) for the PYS measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asakawa, R., Seo, T., Yokoyama, S. et al. A fused π-extended molecule containing an electron-accepting naphthobisthiadiazole and its incorporation into a copolymer: synthesis, properties, and semiconducting performance. Polym J 55, 451–461 (2023). https://doi.org/10.1038/s41428-022-00716-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00716-x