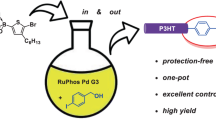
Abstract
In this study, we succeeded in controlling the molecular weight of head-to-tail (HT) regioregular poly(3-hexylthiophene) (P3HT) via palladium-catalyzed direct arylation polymerization (DArP). The key to success was using 2-phenylthiazole (2), which has a highly reactive C–H bond for direct arylation, as the end-capping reagent. The DArP of 2-bromo-3-hexylthiophene (1) in the presence of 2 ([1]0/[2]0 = 10–80), Herrmann-Beller catalyst, and P(o-Me2NC6H4)3 (L1) afforded HT-regioregular P3HT (HT = 99%) capped with a 2-phenylthiazole-5-yl group. The number-average molecular weight (Mn) of P3HT agreed well with the value calculated by assuming formation of one polymer chain per molecule of 2 (polydispersity index: Ð = 1.8–2.1). Thus, the Mn value could be controlled by adjusting the initial molar ratio of 1 to 2. We also succeeded in controlling the molecular weight of poly(3-alkylthiophene)s (alkyl = butyl and octyl) with DArP using 2 as the end-capping reagent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McCullough RD. The chemistry of conducting polythiophenes. Adv Mater. 1998;10:93–116.
Osaka I, McCullough RD. Advances in molecular design and synthesis of regioregular polythiophenes. Acc Chem Res. 2008;41:1202–14.
Agbolaghia S, Zenoozi SA. Comprehensive review on poly(3-alkylthiophene)-based crystalline structures, protocols and electronic applications. Org Electron. 2017;51:362–403.
Guo X, Facchetti A. The journey of conducting polymers from discovery to application. Nat Mater. 2020;19:922–8.
Chen TA, Rieke RD. The first regioregular head-to-tail poly(3-Hexylthiophene-2,5-Diyl) and a regiorandom isopolymer: nickel versus palladium catalysis of 2(5)-bromo-5(2)-(bromozincio)-3-hexylthiophene polymerization. J Am Chem Soc. 1992;114:10087–8.
McCullough RD, Lowe RD. Enhanced electrical conductivity in regioselectively synthesized poly(3-alkylthiophenes). J Chem Soc Chem Commun. 1992;70–2.
Kim Y, Cook S, Tuladhar SM, Choulis SA, Nelson J, Durrant JR, et al. A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene: fullerene solar cells. Nat Mater. 2006;5:197–203.
Woo CH, Thompson BC, Kim BJ, Toney MF, Fréchet JMJ. The influence of poly(3-hexylthiophene) regioregularity on fullerene-composite solar cell performance. J Am Chem Soc. 2008;130:16324–9.
Snyder CR, Henry JS, DeLongchamp DM. Effect of regioregularity on the semicrystalline structure of poly(3-hexylthiophene). Macromolecules. 2011;44:7088–91.
Kohn P, Huettner S, Komber H, Senkovskyy V, Tkachov R, Kiriy A, et al. On the role of single regiodefects and polydispersity in regioregular poly(3-hexylthiophene): defect distribution, synthesis of defect-free chains, and a simple model for the determination of crystallinity. J Am Chem Soc. 2012;134:4790–805.
Kim JS, Kim JH, Lee W, Yu H, Kim HJ, Song I, et al. Tuning mechanical and optoelectrical properties of poly(3-hexylthiophene) through systematic regioregularity control. Macromolecules. 2015;48:4339–46.
Zen A, Pflaum J, Hirschmann S, Zhuang W, Jaiser F, Asawapirom U, et al. Effect of molecular weight and annealing of poly(3-hexylthiophene)s on the performance of organic field-effect transistors. Adv Funct Mater. 2004;14:757–64.
Kline RJ, McGehee MD, Kadnikova EN, Liu J, Frechet JMJ, Toney MF. Dependence of regioregular poly(3-hexylthiophene) film morphology and field-effect mobility on molecular weight. Macromolecules. 2005;38:3312–9.
Schilinsky P, Asawapirom U, Scherf U, Biele M, Brabec CJ. Influence of the molecular weight of poly(3-hexylthiophene) on the performance of bulk heterojunction solar cells. Chem Mater 2005;17:2175–80.
Ballantyne AM, Chen L, Dane J, Hammant T, Braun FM, Heeney M, et al. The effect of poly(3-hexylthiophene) molecular weight on charge transport and the performance of polymer: fullerene solar cells. Adv Funct Mater 2008;18:2373–80.
Koppe M, Brabec CJ, Heiml S, Schausberger A, Duffy W, Heeney M, et al. Influence of molecular weight distribution on the gelation of P3HT and its impact on the photovoltaic performance. Macromolecules. 2009;42:4661–6.
Spoltore D, Vangerven T, Verstappen P, Piersimoni F, Bertho S, Vandewal K, et al. Effect of molecular weight on morphology and photovoltaic properties in P3HT:PCBM solar cells. Org Electron. 2015;21:160–70.
Mardi S, Pea M, Notargiacomo A, Yaghoobi Nia N, Di Carlo A, Reale A. The molecular weight dependence of thermoelectric properties of poly (3-hexylthiophene). Materials. 2020;13:1404.
Yaghoobi Nia N, Bonomo M, Zendehdel M, Lamanna E, Desoky MMH, Paci B, et al. Impact of P3HT regioregularity and molecular weight on the efficiency and stability of perovskite solar cells. ACS Sustain Chem Eng. 2021;9:5061–73.
Yokoyama A, Miyakoshi R, Yokozawa T. Chain-growth polymerization for poly(3-hexylthiophene) with a defined molecular weight and a low polydispersity. Macromolecules. 2004;37:1169–71.
Sheina EE, Liu J, Iovu MC, Laird DW, McCullough RD. Chain growth mechanism for regioregular nickel-initiated cross-coupling polymerizations. Macromolecules. 2004;37:3526–8.
Okamoto K, Luscombe CK. Controlled polymerizations for the synthesis of semiconducting conjugated polymers. Polym Chem 2011;2:2424–34.
Kiriy A, Senkovskyy V, Sommer M. Kumada catalyst-transfer polycondensation: mechanism, opportunities, and challenges. Macromol Rapid Commun. 2011;32:1503–17.
Yokozawa T, Ohta Y. Transformation of step-growth polymerization into living chain-growth polymerization. Chem Rev. 2016;116:1950–68.
Leone AK, Mueller EA, McNeil AJ. The history of palladium-catalyzed cross-couplings should inspire the future of catalyst-transfer. Polymerization J Am Chem Soc. 2018;140:15126–39.
Wang Q, Takita R, Kikuzaki Y, Ozawa F. Palladium-catalyzed dehydrohalogenative polycondensation of 2-bromo-3-hexylthiophene: an efficient approach to head-to-tail poly(3-hexylthiophene). J Am Chem Soc. 2010;132:11420–1.
Wang Q, Wakioka M, Ozawa F. Synthesis of end-capped regioregular poly(3-hexylthiophene)s via direct arylation. Macromol Rapid Commun. 2012;33:1203–7.
Pouliot JR, Wakioka M, Ozawa F, Li Y, Leclerc M. Structural analysis of poly(3-hexylthiophene) prepared via direct heteroarylation polymerization. Macromol Chem Phys. 2016;217:1493–500.
Rudenko AE, Thompson BC. Optimization of direct arylation polymerization (DArP) through the identification and control of defects in polymer structure. J Polym Sci, Part A: Polym Chem. 2015;53:135–47.
Bura T, Blaskovits JT, Leclerc M. Direct (hetero)arylation polymerization: trends and perspectives. J Am Chem Soc. 2016;138:10056–71.
Pouliot JR, Grenier F, Blaskovits JT, Beaupré S, Leclerc M. Direct (hetero)arylation polymerization: simplicity for conjugated polymer synthesis. Chem Rev. 2016;116:14225–74.
Wakioka M, Ozawa F. Highly efficient catalysts for direct arylation polymerization (DArP). Asian J Org Chem. 2018;7:1206–16.
Kuwabara J, Kanbara T. Facile synthesis of π-conjugated polymers via direct arylation polycondensation. Bull Chem Soc Jpn. 2019;92:152–61.
Leclerc M, Brassard S, Beaupré S. Direct (hetero)arylation polymerization: toward defect-free conjugated polymers. Polym J. 2020;52:13–20.
Iizuka E, Wakioka M, Ozawa F. mixed-ligand approach to palladium-catalyzed direct arylation polymerization: synthesis of donor–acceptor polymers with dithienosilole (DTS) and thienopyrroledione (TPD) units. Macromolecules. 2015;48:2989–93.
Iizuka E, Wakioka M, Ozawa F. Mixed-ligand approach to palladium-catalyzed direct arylation polymerization: effective prevention of structural defects using diamines. Macromolecules. 2016;49:3310–7.
Wakioka M, Takahashi R, Ichihara N, Ozawa F. Mixed-ligand approach to palladium-catalyzed direct arylation polymerization: highly selective synthesis of π-conjugated polymers with diketopyrrolopyrrole units. Macromolecules. 2017;50:927–34.
Wakioka M, Nakamura Y, Hihara Y, Ozawa F, Sakaki S. Factors controlling the reactivity of heteroarenes in direct arylation with arylpalladium acetate complexes. Organometallics. 2013;32:4423–30.
Herrmann WA, Brossmer C, Reisinger CP, Riermeier TH, Öfele K, Beller M. Palladacycles: efficient new catalysts for the heck vinylation of aryl halides. Chem Eur J. 1997;3:1357–64.
Fritz HP, Gordon IR, Schwarzhans KE, Venanzi LM. Some complexes of palladium(II) and platinum(II) with mixed phosphorus-nitrogen ligands. J Chem Soc. 1965;5210–6.
Chen CA, Yang PC, Wang SC, Tung SH, Su WF. Side chain effects on the optoelectronic properties and self-assembly behaviors of terthiophene–thieno[3,4-c]pyrrole-4,6-dione based conjugated polymers. Macromolecules. 2018;51:7828–35.
Acknowledgements
This work was supported by KAKENHI (JP24750088, JP15K17855, JP21K05167, and 17H03055) from the Japan Society for the Promotion of Science and the ACT-C program of the Japan Science and Technology Agency. We would like to thank Mr. K. Hatakeyama (Kitasato Univ.) for support in preparing the monomers. We gratefully acknowledge Dr. R. S. Jensen (The Chemical Society of Japan) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wakioka, M., Xu, K., Taketani, T. et al. Synthesis of head-to-tail regioregular poly(3-hexylthiophene)s with controlled molecular weight via highly selective direct arylation polymerization (DArP). Polym J 55, 387–394 (2023). https://doi.org/10.1038/s41428-022-00707-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00707-y