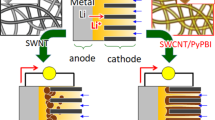
Abstract
Among several strategies employed to reduce overpotential and achieve reliable reversibility with Li–O2 batteries, the use of atomically dispersed bifunctional carbon catalysts is very attractive. However, most of the methods used to prepare these bifunctional oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) catalysts require high temperatures and exhibit low yields, and it is therefore difficult to predetermine the active sites qualitatively and quantitatively. Here, we propose the use of atomically dispersed metal centers coordinated to diimine moieties of conjugated polymers as bifunctional catalysts without further modification (pyrolysis or composite formation) for Li–O2 battery applications. Poly(bisiminoacenaphthenequinone) (BIAN) iron complex (BP-Fe) catalysts showed high OER activities, which enabled 100% coulombic efficiency for 160 galvanostatic charge discharge cycles with a capacity limit of 500 mAh/g at a current density of 250 mA/g. The overpotential corresponding to charging was as low as ~1.0 V and exhibited almost no change in discharge overpotential across 160 cycles. Additionally, it showed a commendable rate capability with only a 170 mV increase in charge overpotential when the charge‒discharge rate was increased from 100 to 500 mA/g.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
References
Aurbach D, McCloskey BD, Nazar LF, Bruce PG. Advances in understanding mechanisms underpinning lithium–air batteries. Nat Energy. 2016;1:1–11.
Zhang P, Zhao Y, Zhang X. Functional and stability orientation synthesis of materials and structures in aprotic Li–O2 batteries. Chem Soc Rev. 2018;47:2921–3004.
Abraham KM, Jiang Z. A polymer electrolyte‐based rechargeable lithium/oxygen battery. J Electrochem Soc. 1996;143:1.
Bruce PG, Freunberger SA, Hardwick LJ, Tarascon JM. Li–O2 and Li–S batteries with high energy storage. Nat Mat. 2012;11:19–29.
Laoire CO, Mukerjee S, Abraham KM, Plichta EJ, Hendrickson MA. Elucidating the mechanism of oxygen reduction for lithium-air battery applications. J Phys Chem C. 2009;113:20127–34.
Lu YC, Gallant BM, Kwabi DG, Harding JR, Mitchell RR, Whittingham MS, et al. Lithium–oxygen batteries: bridging mechanistic understanding and battery performance. Energy Environ Sci. 2013;6:750–68.
Xia CY, Kwok CY, Nazar LF. A high-energy-density lithium-oxygen battery based on a reversible four-electron conversion to lithium oxide. Science. 2018;361:777–81.
Zhang Y, Wang L, Zhang X, Guo L, Wang Y, Peng Z. High‐capacity and high‐rate discharging of a coenzyme Q10‐catalyzed Li–O2 battery. Adv Mater 2018;30:1705571.
Lin X, Yuan R, Cao Y, Ding X, Cai S, Han B, et al. Controlling reversible expansion of Li2O2 formation and decomposition by modifying electrolyte in Li-O2 batteries. Chem. 2018;4:2685–98.
Ko Y, Park H, Kim B, Kim JS, Kang K. Redox mediators: a solution for advanced lithium–oxygen batteries. Trends Chem. 2019;1:349–60.
Nakanishi A, Thomas ML, Kwon HM, Kobayashi Y, Tatara R, Ueno K, et al. Electrolyte composition in Li/O2 batteries with LiI redox mediators: solvation effects on redox potentials and implications for redox shuttling. J Phys Chem C. 2018;122:1522–34.
Yilmaz E, Yogi C, Yamanaka K, Ohta T, Byon HR. Promoting formation of noncrystalline Li2O2 in the Li–O2 battery with RuO2 nanoparticles. Nano Lett. 2013;13:4679–84.
McCloskey BD, Speidel A, Scheffler R, Miller DC, Viswanathan V, Hummelshøj JS, et al. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J Phys Chem Lett. 2012;3:997–1001.
Yao KP, Risch M, Sayed SY, Lee YL, Harding JR, Grimaud A, et al. Solid-state activation of Li2O2 oxidation kinetics and implications for Li–O2 batteries. Energy Environ Sci. 2015;8:2417–26.
Radin MD, Monroe CW, Siegel DJ. How dopants can enhance charge transport in Li2O2. Chem Mater. 2015;27:839–47.
Black R, Lee JH, Adams B, Mims CA, Nazar LF. The role of catalysts and peroxide oxidation in lithium–oxygen batteries. Angew Chem Int Ed. 2013;52:392–6.
Wang Y, Liang Z, Zou Q, Cong G, Lu YC. Mechanistic insights into catalyst-assisted nonaqueous oxygen evolution reaction in lithium–oxygen batteries. J Phys Chem C. 2016;120:6459–66.
McCloskey BD, Addison D. A viewpoint on heterogeneous electrocatalysis and redox mediation in nonaqueous Li-O2 batteries. ACS Catal. 2017;7:772–8.
Mahne N, Fontaine O, Thotiyl MO, Wilkening M, Freunberger SA. Mechanism and performance of lithium–oxygen batteries–a perspective. Chem Sci. 2017;8:6716–29.
McCloskey BD, Scheffler R, Speidel A, Bethune DS, Shelby RM, Luntz AC. On the efficacy of electrocatalysis in nonaqueous Li–O2 batteries. J Am Chem Soc. 2011;133:18038–41.
Ganapathy S, Adams BD, Stenou G, Anastasaki MS, Goubitz K, Miao XF, et al. Nature of Li2O2 oxidation in a Li–O2 battery revealed by operando X- ray diffraction. J Am Chem Soc. 2014;136:16335–44.
Harding JR, Lu YC, Tsukada Y, Shao-Horn Y. Evidence of catalyzed oxidation of Li2O2 for rechargeable Li–air battery applications. Phys Chem Chem Phys. 2012;14:10540–6.
Lu YC, Xu Z, Gasteiger HA, Chen S, Hamad-Schifferli K, Shao-Horn Y. Platinum−gold nanoparticles: a highly active bifunctional electrocatalyst for rechargeable lithium−air batteries. J Am Chem Soc. 2010;132:12170–1.
Lu J, Lei Y, Lau KC, Luo X, Du P, Wen J, et al. A nanostructured cathode architecture for low charge overpotential in lithium-oxygen batteries. Nat Commun. 2013;4:2383.
Trahey L, Karan NK, Chan MK, Lu J, Ren Y, Greeley J, et al. Synthesis, characterization, and structural modeling of high‐capacity, dual functioning MnO2 electrode/electrocatalysts for Li‐O2 cells. Adv Energy Mater. 2013;3:75–84.
Débart A, Paterson AJ, Bao J, Bruce PG. α‐MnO2 nanowires: a catalyst for the O2 electrode in rechargeable lithium batteries. Angew Chem Int Ed. 2008;47:4521–4.
Débart A, Bao J, Armstrong G, Bruce PG. An O2 cathode for rechargeable lithium batteries: the effect of a catalyst. J Power Sources. 2007;174:1177–82.
Qin Y, Lu J, Du P, Chen Z, Ren Y, Wu T, et al. In situ fabrication of porous-carbon-supported α-MnO2 nanorods at room temperature: application for rechargeable Li–O2 batteries. Energy Environ Sci. 2013;6:519–31.
Hegde GS, Ghosh A, Badam R, Matsumi N, Sundara R. Role of defects in low-cost perovskite catalysts toward ORR and OER in lithium–oxygen batteries. ACS Appl Energy Mater. 2020;3:1338–48.
Paraknowitsch JP, Thomas A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ Sci. 2013;6:2839–55.
Li Y, Wang J, Li X, Geng D, Banis MN, Tang Y, et al. Discharge product morphology and increased charge performance of lithium–oxygen batteries with graphene nanosheet electrodes: the effect of sulphur doping. J Mat Chem. 2012;22:20170–4.
Kim JH, Kannan AG, Woo HS, Jin DG, Kim W, Ryu K, et al. A bi-functional metal-free catalyst composed of dual-doped graphene and mesoporous carbon for rechargeable lithium–oxygen batteries. J Mat Chem A. 2015;3:18456–65.
Shu C, Lin Y, Su D. N-doped onion-like carbon as an efficient oxygen electrode for long-life Li–O2 battery. J Mat Chem A. 2016;4:2128–36.
Wu F, Xing Y, Li L, Qian J, Qu W, Wen J, et al. Facile synthesis of boron-doped rGO as cathode material for high energy Li–O2 batteries. ACS Appl Mater Interfaces. 2016;8:23635–45.
Patnaik KS, Badam R, Peng Y, Higashimine K, Kaneko T, Matsumi N. Extremely fast charging lithium-ion battery using bio-based polymer-derived heavily nitrogen doped carbon. Chem Commun. 2021;57:13704–7.
Cui Y, Wen Z, Liang X, Lu Y, Jin J, Wu M, et al. A tubular polypyrrole based air electrode with improved O 2 diffusivity for Li–O2 batteries. Energy Environ Sci. 2012;5:7893–7.
Weng W, Barile CJ, Du P, Abouimrane A, Assary RS, Gewirth AA, et al. Polymer supported organic catalysts for O2 reduction in Li-O2 batteries. Electrochim Acta. 2014;119:138–43.
Lu Q, Zhao Q, Zhang H, Li J, Wang X, Wang F. Water dispersed conducting polyaniline nanofibers for high-capacity rechargeable lithium–oxygen battery. ACS Macro Lett. 2013;19:92–5.
Patnaik SG, Vedarajan R, Matsumi N. BIAN based electroactive polymer with defined active centers as metal-free electrocatalysts for oxygen reduction reaction (ORR) in aqueous and nonaqueous media. ACS Appl Energy Mater. 2018;1:1183–90.
Hu X, Luo G, Zhao Q, Wu D, Yang T, Wen J, et al. Ru single atoms on N-doped carbon by spatial confinement and ionic substitution strategies for high- performance Li–O2 batteries. J Am Chem Soc. 2020;142:16776–86.
Lei X, Liu B, Koudakan PA, Pan H, Qian Y, Wang G. Single-atom catalysts cathode for lithium-Oxygen batteries: a review. Nano Futures. 2021;6:012002.
Asikin-Mijan N, Mohd Sidek H, AlSultan AG, Azman NA, Adzahar NA, Ong HC. Single-atom catalysts: a review of synthesis strategies and their potential for biofuel production. Catalysts. 2021;11:1470.
Yu X, Zhu F, Bu D, Lei H. Ferrous complexes supported by sterically encumbered asymmetric bis (arylimino) acenaphthene (BIAN) ligands: synthesis, characterization and screening for catalytic hydrosilylation of carbonyl compounds. RSC Adv. 2017;7:15321–9.
Gupta A, Badam R, Nag A, Kaneko T, Matsumi N. Bis-imino-acenaphthenequinone- paraphenylene-type condensation copolymer binder for ultralong cyclable lithium-ion rechargeable batteries. ACS Appl Energy Mater. 2021;4:2231–40.
Gupta A, Badam R, Matsumi N. Heavy-duty performance from silicon anodes using poly(BIAN)/poly(acrylic acid)-based self-healing composite binder in lithium-ion secondary batteries. ACS Appl Energy Mater. 2022. https://doi.org/10.1021/acsaem.2c00278.
Zhang C, Ma J, Han F, Liu H, Zhang F, Fan C, et al. Strong anchoring effect of ferric chloride-graphite intercalation compounds (FeCl3-GICs) with tailored epoxy groups for high-capacity and stable lithium storage. J Mater Chem A. 2018;6:17982–93.
Peng Z, Freunberger SA, Hardwick LJ, Chen Y, Giordani V, Bardé F, et al. Oxygen reactions in a non‐aqueous Li+ electrolyte. Angew Chem Int Ed. 2011;50:6351–5.
Ryu WH, Gittleson FS, Thomsen JM, Li J, Schwab MJ, Brudvig GW, et al. Heme biomolecule as redox mediator and oxygen shuttle for efficient charging of lithium-oxygen batteries. Nat Comm. 2016;19:1–10.
Asadi M, Sayahpour B, Abbasi P, Ngo AT, Karis K, Jokisaari JR, et al. A lithium–oxygen battery with a long cycle life in an air- like atmosphere. Nature. 2018;555:502–6.
Yao KP, Kwabi DG, Quinlan RA, Mansour AN, Grimaud A, Lee YL, et al. Thermal stability of Li2O2 and Li2O for Li-air batteries: in situ XRD and XPS studies. J Electrochem Soc. 2013;160:A824.
Ma Q, Tong B, Fang Z, Qi X, Feng W, Nie J, et al. Impact of anionic structure of lithium salt on the cycling stability of lithium-metal anode in Li-S batteries. J Electrochem Soc. 2016;163:A1776.
Acknowledgements
The authors are thankful to the Centre for Nano Materials and Technology (CNMT) at JAIST for providing the characterization facilities. We are thankful for the financial support from JSPS Kakenhi Grant Number JP21K14714 and MEXT Elements Strategy Initiative for Catalysts and Batteries (JPMXP0112101003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Badam, R., Shibuya, M., Mantripragada, B.S. et al. BIAN-based durable polymer metal complex as a cathode material for Li–O2 battery applications. Polym J 54, 1355–1366 (2022). https://doi.org/10.1038/s41428-022-00699-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00699-9