Abstract
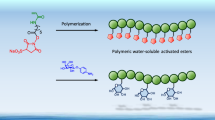
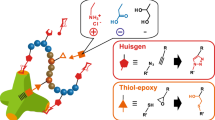
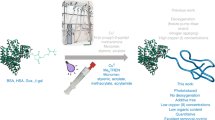
In this focus review, recent developments in unnatural sugar- and protein-based polymers and their future bioapplications are discussed. A new unnatural oligoaminosaccharide carrying N-1,2-glycosidic bonds that cannot be prepared in natural biological systems has been proposed. To prepare the oligomers, a sugar monomer possessing a 2-methyl-2-oxazoline (MeOx) ring was polymerized via cationic ring-opening polymerization. This polymerization did not proceed by the classical MeOx mechanism but by a new mechanism involving sequential SN1-type reactions. This unnatural oligosaccharide was not decomposed by the natural enzymes owing to the unnatural N-1,2-glycosidic bonds, indicating promise in applications as a new class of glycomaterials. Furthermore, technology for stabilizing proteins using protein–polymer conjugations and polymer chain-folding nanoparticles has recently been developed. Amphiphilic/fluorous methacrylate-based random copolymers bearing polyethylene glycol (PEG) and fluorous side chains formed reversible PEG and fluorous compartments in water and 2H,3H-perfluoropentane (2HPFP), respectively. These copolymers were noncytotoxic and successfully conjugated with lysozymes. They also stabilized lysozyme and α-chymotrypsin in 2HPFP, and the enzymes were not denatured after extraction from 2HPFP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Imberty A, Varrot A. Microbial recognition of human cell surface glycoconjugates. Curr Opin Struct Biol. 2008;18:567–76.
Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14:569–76.
Lee YC, Lee RT. Carbohydrate–protein interactions: basis of glycobiology. Acc Chem Res. 1995;28:321–7.
O’Connor SE, Imperiali B. A molecular basis for glycosylation-induced conformational switching. Chem Biol. 1998;5:427–37.
Hipp MS, Park SH, Ulrich Hartl F. Proteostasis impairment in protein- misfolding and -aggregation diseases. Trends Cell Biol. 2014;24:506–14.
Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem. 2017;86:27–68.
Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703–19.
Liu Q, Chen G, Chen H. Chemical synthesis of glycosaminoglycan-mimetic polymers. Polym Chem. 2019;10:164–71.
Nguyen TH, Kim SH, Decker CG, Wong DY, Loo JA, Maynard HD. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat Chem. 2013;5:221–7.
Ando M, Akiyama M, Okuno D, Hirano M, Ide T, Sawada S, et al. Liposome chaperon in cell-free membrane protein synthesis: one-step preparation of KcsA-integrated liposomes and electrophysiological analysis by the planar bilayer method. Biomater Sci. 2016;4:258–64.
Ando M, Schikula S, Sasaki Y, Akiyoshi K. Proteoliposome engineering with cell-free membrane protein synthesis: control of membrane protein sorting into liposomes by chaperoning systems. Adv Sci. 2018;5:1800524.
Nourian Z, Roelofsen W, Danelon C. Triggered gene expression in fed-vesicle microreactors with a multifunctional membrane. Angew Chem Int Ed. 2012;51:3114–8.
Palivan CG, Goers R, Najer A, Zhang X, Cara A, Meier W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem Soc Rev. 2016;45:377–411.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, Kataoka K, et al. Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res. 1990;50:1693–700.
Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed. 2003;42:4640–3.
Liu D, Wang T, Lu Y. Untethered microrobots for active drug delivery: from rational design to clinical settings. Adv Healthc Mater. 2022;11:2102253.
Mi P, Cabral H, Kataoka K. Ligand-installed nanocarriers toward precision therapy. Adv Mater. 2020;32:1902604.
Qi W, Zhang Y, Wang J, Tao G, Wu L, Kochovski Z, et al. Deprotection-induced morphology transition and immunoactivation of glycovesicles: a strategy of smart delivery polymersomes. J Am Chem Soc. 2018;140:8851–7.
Yang J, Du Q, Li L, Wang T, Feng Y, Nieh MP, et al. Glycosyltransferase-induced morphology transition of glycopeptide self-assemblies with proteoglycan residues. ACS Macro Lett. 2020;9:929–36.
Nishimura T, Hirose S, Sasaki Y, Akiyoshi K. Substrate-sorting nanoreactors based on permeable peptide polymer vesicles and hybrid liposomes with synthetic macromolecular channels. J Am Chem Soc. 2020;142:154–61.
Vong LB, Trinh NT, Nagasaki Y. Design of amino acid-based self-assembled nano-drugs for therapeutic applications. J Control Release. 2020;326:140–9.
Kudo S, Nagasaki Y. A novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(L-arginine)-based nanoparticles. J Control Release. 2015;217:256–62.
Vong LB, Sato Y, Chonpathompikunlert P, Tanasawet S, Hutamekalin P, Nagasaki Y. Self-assembled polydopamine nanoparticles improve treatment in Parkinson’s disease model mice and suppress dopamine-induced dyskinesia. Acta Biomater. 2020;109:220–8.
Shashni B, Tajika Y, Nagasaki Y. Design of enzyme-responsive short-chain fatty acid-based self-assembling drug for alleviation of type 2 diabetes mellitus. Biomaterials. 2021;275:120877.
Koda Y, Terashima T, Ouchi M. Unnatural oligoaminosaccharides with N-1,2-glycosidic bonds prepared by cationic ring-opening polymerization of 2-oxazoline-based heterobicyclic sugar monomers. ACS Macro Lett. 2019;8:1456–60.
Koda Y, Terashima T, Sawamoto M, Maynard HD. Amphiphilic/fluorous random copolymers as a new class of non-cytotoxic polymeric materials for protein conjugation. Polym Chem. 2015;6:240–7.
Koda Y, Terashima T, Maynard HD, Sawamoto M. Protein storage with perfluorinated PEG compartments in a hydrofluorocarbon solvent. Polym Chem. 2016;7:6694–8.
Abuchowski A, Mccoy JR, Palczuk NC, Es TV, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582–6.
Messina MS, Messina KMM, Bhattacharya A, Montgomery HR, Maynard HD. Preparation of biomolecule-polymer conjugates by grafting-from using ATRP, RAFT, or ROMP. Prog Polym Sci. 2020;100:101186.
Tamura T, Hamachi I. Chemistry for covalent modification of endogenous/native proteins: from test tubes to complex biological systems. J Am Chem Soc. 2019;141:2782–99.
Koda Y, Terashima T, Sawamoto M. LCST-type phase separation of poly[poly(ethylene glycol) methyl ether methacrylate]s in hydrofluorocarbon. ACS Macro Lett. 2015;4:1366–9.
Koda Y, Terashima T, Sawamoto M. Multimode self-folding polymers via reversible and thermoresponsive self-assembly of amphiphilic/fluorous random copolymers. Macromolecules. 2016;49:4534–43.
Ihsan AB, Koyama Y. Substituent optimization of (1→2)-glucopyranan for tough, strong, and highly stretchable film with dynamic interchain interactions. ACS Macro Lett. 2020;9:720–4.
Dey S, Lo HJ, Wong CH. An efficient modular one-pot synthesis of heparin-based anticoagulant idraparinux. J Am Chem Soc. 2019;141:10309–14.
Tanaka M, Sato K, Yoshida R, Nishi N, Oyamada R, Inaba K, et al. Diastereoselective desymmetric 1,2-cis-glycosylation of meso-diols via chirality transfer from a glycosyl donor. Nat Commun. 2020;11:2431.
Hettikankanamalage AA, Lassfolk R, Ekholm FS, Leino R, Crich D. Mechanisms of stereodirecting participation and ester migration from near and far in glycosylation and related reactions. Chem Rev. 2020;120:7104–51.
Kinnaert C, Daugaard M, Nami F, Clausen MH. Chemical synthesis of oligosaccharides related to the cell walls of plants and algae. Chem Rev. 2017;117:11337–405.
Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Ann Rev Biochem. 1988;57:785–838.
Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291:2357–64.
Miura Y, Hoshino Y, Seto H. Glycopolymer nanobiotechnology. Chem Rev. 2016;116:1673–92.
Hu Y, Li Y, Xu FJ. Versatile functionalization of polysaccharides via polymer grafts: from design to biomedical applications. Acc Chem Res. 2017;50:281–92.
Su L, Feng Y, Wei K, Xu X, Liu R, Chen G. Carbohydrate-based macromolecular biomaterials. Chem Rev. 2021;121:10950–1029.
Tahara Y, Akiyoshi K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv Drug Deliv Rev. 2015;95:65–76.
Heinzea T, Liebert T. Unconventional methods in cellulose functionalization. Prog Polym Sci. 2001;26:1689–762.
Kadokawa J. Precision polysaccharide synthesis catalyzed by enzymes. Chem Rev. 2011;111:4308–45.
Chen G. The past ten years of carbohydrate polymers in ACS Macro Letters. ACS Macro Lett. 2021;10:1145–50.
Ichikawa H, Kobayashi K, Sumitomo H, Schuerch C. Synthesis of A β-(1→6)-linked polysaccharide via ring-opening polymerization with neighboring-group participation. Carbohydr Res. 1988;179:315–20.
Okada M, Kubota Y. Chemical synthesis of polysaccharides XI. ring-opening polymerization of 1,6-anhydro-2-O-(p-substituted benzoyl) deoxysugar derivatives. Polym J. 1992;24:1137–45.
Kobayashi K, Ishii T, Okada M, Schuerch C. Steric control in ring-opening polymerization of 1,6-anhydro galactose derivatives by neighboring group participation. Polym J. 1993;25:49–57.
Stidham SE, Chin SL, Dane EL, Grinstaff MW. Carboxylated glucuronic poly-amido-saccharides as protein stabilizing agents. J Am Chem Soc. 2014;136:9544–7.
Xiao R, Dane EL, Zeng J, McKnight CJ, Grinstaff MW. Synthesis of altrose poly-amido-saccharides with β-N‐(1→2)‐D‐amide linkages: a right-handed helical conformation engineered in at the monomer level. J Am Chem Soc. 2017;139:14217–23.
Su L, Khan S, Fan J, Lin YN, Wang H, Gustafson TP, et al. Functional sugar-based polymers and nanostructures comprised of degradable poly(D-glucose carbonate)s. Polym Chem. 2017;8:1699–707.
Felder SE, Redding MJ, Noel A, Grayson SM, Wooley KL. Organocatalyzed ROP of a glucopyranoside derived five-membered cyclic carbonate. Macromolecules. 2018;51:1787–97.
Deleray A, Kramer JR. Biomimetic glycosylated polythreonines by N-carboxyanhydride polymerization. Biomacromolecules. 2022;23:1453–61.
Mancini RJ, Lee J, Maynard HD. Trehalose glycopolymers for stabilization of protein conjugates to environmental stressors. J Am Chem Soc. 2012;134:8474–9.
Ishida T, Ichikawa T, Ichihara M, Sadzuka Y, Kiwada H. Effect of the physicochemical properties of initially injected liposomes on the clearance of subsequently injected PEGylated liposomes in mice. J Control Release. 2004;95:403–12.
Lila ASS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Contol Release. 2013;172:38–47.
Nagai R, Mori T, Yamamoto Y, Kaji Y, Yonei Y. Significance of advanced glycation end products in aging-related disease. Anti-Aging Med. 2010;7:112–9.
Aragonès G, Rowan S, Francisco SG, Yang W, Weinberg J, Taylor A, et al. Glyoxalase system as a therapeutic target against diabetic retinopathy. Antioxidants. 2020;9:1062.
Shimizu H, Ohue-Kitano R, Kimura I. Regulation of host energy metabolism by gut microbiota-derived short-chain fatty acids. Glycative Stress Res. 2019;6:181–91.
de Kort BJ, Koch SE, Wissing TB, Krebber MM, Bouten CVC, Smits AIPM. Immuno-regenerative biomaterials for in situ cardiovascular tissue engineering – Do patient characteristics warrant precision engineering? Adv Drug Deliv Rev. 2021;178:113960.
Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL. Mechanisms of diabetes mellitus- induced bone fragility. Nat Rev Endocrinol. 2017;13:208–19.
Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, et al. Advanced glycation end products contribute to amyloidosis in alzheimer disease. Proc Natl Acad Sci USA. 1994;91:4766–70.
Takahashi N, Harada M, Azhary JMK, Kunitomi C, Nose E, Terao H, et al. Accumulation of advanced glycation end products in follicles is associated with poor oocyte developmental competence. Mol Hum Reprod. 2019;25:684–94.
Fehl C, Hanover JA. Tools, tactics and objectives to interrogate cellular roles of O-GlcNAc in disease. Nat Chem Biol. 2022;18:8–17.
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38.
Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Deliv Rev. 2012;64:194–205.
Dutta K, Hu D, Zhao B, Ribbe AE, Zhuang J, Thayumanavan S. Templated self-assembly of a covalent polymer network for intracellular protein delivery and traceless release. J Am Chem Soc. 2017;139:5676–9.
Rose DA, Treacy JW, Yang ZJ, Ko JH, Houk KN, Maynard HD. Self-immolative hydroxybenzylamine linkers for traceless protein modification. J Am Chem Soc. 2022;144:6050–8.
Sasaki Y, Akiyoshi K. Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem Rec. 2010;10:366–76.
Harada M, Takahashi N, Mk Azhary J, Kunitomi C, Fujii T, Osuga Y. Endoplasmic reticulum stress: a key regulator of the follicular microenvironment in the ovary. Mol Hum Reprod. 2021;27:gaaa088.
Horváth IT, Rábai J. Facile catalyst separation without water: fluorous biphase hydroformylation of olefins. Science. 1994;266:72–5.
Yao H, Sheng K, Sun J, Yan S, Hou Y, Lu H, et al. Secondary structure drives self-assembly in weakly segregated globular protein–rod block copolymers. Polym Chem. 2020;11:3032–45.
Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18:285–98.
Franzmann TM, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse AS, Nüske E, et al. Phase separation of a yeast prion protein promotes cellular fitness. Science. 2018;359:eaao5654.
Harris IS, DeNicola GM. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020;30:440–51.
Koda Y, Terashima T, Nomura A, Ouchi M, Sawamoto M. Fluorinated microgel-core star polymers as fluorous compartments for molecular recognition. Macromolecules. 2011;44:4574–8.
Koda Y, Terashima T, Sawamoto M. Fluorous microgel star polymers: selective recognition and separation of polyfluorinated surfactants and compounds in water. J Am Chem Soc. 2014;136:15742–8.
Ouchi M, Terashima T, Sawamoto M. Transition metal-catalyzed living radical polymerization: toward perfection in catalysis and precision polymer synthesis. Chem Rev. 2009;109:4963–5050.
Koda Y, Terashima T, Takenaka M, Sawamoto M. Star polymer gels with fluorinated microgels via star–star coupling and cross-linking for water purification. ACS Macro Lett. 2015;4:377–80.
Koda Y, Terashima T, Sawamoto M. Fluorinated microgel star polymers as fluorous nanocapsules for the encapsulation and release of perfluorinated compounds. Polym Chem. 2015;6:5663–74.
Koda Y, Terashima T, Sawamoto M. Fluorinated microgels in star polymers: from in-core dynamics to fluorous encapsulation. Macromolecules. 2015;48:2901–8.
Acknowledgements
The author appreciates the support of the Japan Society for the Promotion of Science (JSPS) KAKENHI for this research through a Grant-in-Aid for Young Scientists (B, 17K14494; 20K15336) for the unnatural saccharide project. The author further thanks his supervisors in the protein projects, Prof. Mituso Sawamoto (Chubu Univ.), Prof. Takaya Terashima (Kyoto Univ.) and Prof. Heather D. Maynard (University of California, Los Angeles; UCLA), for their kind support and guidance. The author is also grateful to JSPS for a Grant-in-Aid for JSPS Research Fellows (DC1: 24-6140) for his fluorous projects. The author would like to thank AJE for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koda, Y. Unnatural biopolymers of saccharides and proteins conjugated with poly(2-oxazoline) and methacrylate-based polymers: from polymer design to bioapplication. Polym J 54, 1431–1444 (2022). https://doi.org/10.1038/s41428-022-00695-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00695-z