Abstract
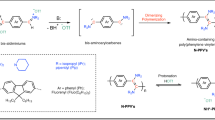
Poly(2,5-dimethyl-1,4-phenylenesulfide) (PMPS) was prepared without halogenated compounds via oxygen oxidative polymerization of bis(2,5-dimethylphenyl) disulfide (2,5-DPS) with a VO(acac)2-strong acid catalyst under bulk conditions. The polymer showed crystalline features with a high melting temperature (Tm = 268 °C). After the subsequent heat curing of PMPS through the exchange reaction of a reactive disulfide bond, the polymer had a higher melting temperature (Tm = 286 °C), which was even higher than that of conventional poly(p-phenylene sulfide) (PPS) (Tm = 283 °C). Furthermore, the copolymerization of 2,5-DPS with diphenyl disulfide (DPS) led to products with higher crystallinities and melting temperatures owing to the increase in their molecular weights, which was derived from these products having higher solubilities in the monomer melt than PMPS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Zuo P, Tcharkhtchi A, Shirinbayan M, Fitoussi J, Bakir F. Overall Investigation of Poly (Phenylene Sulfide) from Synthesis and Process to Applications—A Review. Macromol Mater Eng. 2019;304:1800686.
Kanomata A, Horiuchi S, Kaiho S, Yamauchi K U.S. Patent 9000104 B2. 2014.
Aida F, Takasu N, Takatori Y, Nishide H, Oyaizu K. Synthesis of Highly Crystallized Poly(1,4-phenylene sulfide) via Oxygen-Oxidative Polymerization of Diphenyl Disulfide. Bull Chem Soc Jpn. 2017;90:843–6.
Yamamoto K, Tsuchida E, Nishide H, Jikei M, Oyaizu K. Oxovanadium-Catalyzed Oxidative Polymerization of Diphenyl Disulfides with Oxygen. Macromolecules. 1993;26:3432–7.
Watanabe S, Oyaizu K. Methoxy-Substituted Phenylenesulfide Polymer with Excellent Dispersivity of TiO2 Nanoparticles for Optical Application. Bull Chem Soc Jpn. 2020;93:1287–92.
Hay A. Poly(phenylene oxide)s and poly(arylene ether)s derived from 2,6-diarylphenols. Prog Polym Sci. 1999;24:45–80.
Oyaizu K, Kumaki Y, Saito K, Tsuchida E. First synthesis of high molecular weight poly(2,6-difluoro-l,4-phenylene oxide) by oxidative polymerization. Macromolecules. 2000;33:5766–9.
Saito K, Tazaki T, Matsubara R, Nishide H. Acid-Functionalized Poly(phenylene oxide)s: their preparation and properties. Ind Eng Chem Res. 2005;44:8626–30.
Higashimura H, Fujisawa K, Moro-oka Y, Kubota M, Shiga A, Terahara A, Uyama H, Kobayashi S. Highly Regioselective Oxidative Polymerization of 4-Phenoxyphenol to Poly(1,4-phenylene oxide) Catalyzed by Tyrosinase Model Complexes. J Am Chem Soc. 1998;120:8529–30.
Higashimura H, Fujisawa K, Moro-oka Y, Namekawa S, Kubota M, Shiga A, Uyama H, Kobayashi S. New crystalline polymers: poly(2,5-dialkyl-1,4-phenylene oxide)s. Macromol Rapid Commun. 2000;21:1121–4.
Shibasaki Y, Nakamura M, Ishimaru R, Kondo JN, Domen K, Ueda M. Regiocontrolled Oxidative Coupling Polycondensation of 2,5-Dimethylphenol Induced by Mesoporous Interior. Macromolecules. 2004;37:9657–9.
Shibasaki Y, Suzuki Y, Ueda M. Copper-Catalyzed Regio-Controlled Oxidative Coupling Polymerization of 2,5-Dimethylphenol. Macromolecules. 2007;40:5322–5.
Aiba M, Sun XC, Shibasaki Y, Oda M, Ooka C, Fukuta S, Higashihara T, Ando S, Ueda M, Chen WC. Synthesis of poly(o-cresol) by oxidative coupling polymerization of o-cresol. J Polym Sci Part A Polym Chem. 2019;57:878–84.
Shibasaki Y, Hatusgai S, Tsukamoto T, Oishi Y. Synthesis of Poly(thymol) via Oxidative Coupling Polymerization. J Photopolym Sci Technol. 2020;33:301–6.
Aida F, Takatori Y, Kiyokawa D, Nagamatsu K, Oyaizu K, Nishide H. Enhanced catalytic activity of oxovanadium complexes in oxidative polymerization of diphenyl disulfide. Polym Chem. 2016;7:2087–91.
Shouji E, Yamamoto K, Katoh J, Nishide H, Tsuchida E. Effect of substituent groups of diphenyl disulfide on novel cationic oxidative polymerization: examination of the electrophilic reaction of the cation by computational calculation. Polym Adv Technol. 1991;2:149–54.
Aida F, Takatori Y, Kiyokawa D, Nagamatsu K, Nishide H, Oyaizu K. Poly(1,4-phenylene sulfide) (PPS) synthesis via oxidative polymerization of diphenyl disulfide: Mechanistic insight into the selective formation of 1,4-thiophenylene chain. Chem Lett. 2015;44:767–9.
Jikei M, Katoh J, Sato N, Yamamoto K, Nishide H, Tsuchida E. Synthesis of Soluble Poly(thio-2,6-dimethyl-1,4-phenylene) by Oxidative Polymerization of Bis(3,5-dimethylphenyl) Disulfide. Bull Chem Soc Jpn. 1992;65:2029–36.
Watanabe S, Oyaizu K. Catechol End-Capped Poly(arylene sulfide) as a High-Refractive-Index “TiO2/ZrO2-Nanodispersible” Polymer. ACS Appl Polym Mater. https://doi.org/10.1021/acsapm.1c00536 (in press).
Miyatake K, Jikei M, Katoh J, Yamamoto K, Nishide H, Tsuchida E. Synthesis of poly(2,5-dimethylphenylene sulfide) through oxidative polymerization of sulfur chloride with p-xylene. Polym Adv Technol. 1994;5:216–20.
Yamamoto K, Jikei M, Katoh J, Nishide H, Tsuchida E. Synthesis of Poly(arylene sulfide)s by Cationic Oxidative Polymerization of Diaryl Disulfides. Macromolecules. 1992;25:2698–704.
Aida F, Yamaguchi S, Takatori Y, Nagamatsu K, Kiyokawa D, Oyaizu K, Nishide H. Vanadyl-TrBR4-Catalyzed Oxidative Polymerization of Diphenyl Disulfide. Macromol Chem Phys. 2015;216:1850–5.
Azeyanagi C, Ohki Y. Terahertz spectroscopic estimation of crystallinity of poly(phenylene sulfide). J Appl Polym Sci. 2018;135:46427.
Housaki T, Satoh K. Molecular Weight Distribution of Polyphenylene Sulfide by High Temperature Gel Permeation Chromatography. Polym J. 1988;20:1163–6.
Tsuchida E, Yamamoto K, Oyaizu K, Suzuki F, Hay AS, Wang ZY. Synthesis of Reactive Functionalized Oligo(p-phenylene sulfide)s. Macromolecules. 1995;28:409–16.
Thakur KAM, Kean RT, Zupfer JM, Buehler NU, Doscotch MA, Munson EJ. Solid State 13C CP-MAS NMR Studies of the Crystallinity and Morphology of Poly(l-lactide). Macromolecules. 1996;29:8844–51.
Acknowledgements
This work was partially supported by the Grants-in-Aid for Scientific Research (Nos. 21H04695 and 18H05515) from MEXT, Japan. We thank Dr Toshimichi Shibue (Materials Characterization Central Laboratory, Waseda University) for the solid-state NMR measurements. This work was the result of using research equipment (NMR spectrometer) in the Materials Characterization Central Laboratory of Waseda University, which was shared in the MEXT Project for promoting public utilization of advanced research infrastructure (program for supporting the construction of core facilities, Grant No. JPMXS0440500021).
Author information
Authors and Affiliations
Contributions
All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Watanabe, S., Saito, S., Hirai, M. et al. Synthesis of methylated phenylene sulfide polymers via bulk oxidative polymerization and their heat curing triggered by dynamic disulfide exchange. Polym J 54, 1–10 (2022). https://doi.org/10.1038/s41428-021-00557-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00557-0