Abstract
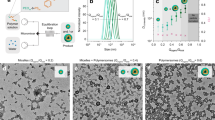
Thermoresponsive polymer vesicles are promising drug-delivery carriers because of their ability to release cargo in a controlled manner. However, the process of thermoresponsive vesicle disassembly has attracted only limited attention to date, and the details remain elusive. Herein, we report using small-angle X-ray scattering and confocal laser scanning microscopy for in situ observation of the disassembly process during the cooling of thermoresponsive maltopentaose-b-poly(propylene oxide) polymer vesicle solutions. Notably, vesicle collapse mainly involves three discrete steps, not a concerted process. We also demonstrate that protein-loaded vesicles can be triggered to release their cargo upon cooling and that released protein retains its enzymatic activity. This study can thus provide a basis for generating guidelines for using thermoresponsive polymer vesicles in the triggered release of cargo and facilitating the development of new thermoresponsive materials based on poly(propylene oxide).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297:967. https://doi.org/10.1126/science.1074972
Antonietti M, Förster S. Vesicles and liposomes: a self-assembly principle beyond lipids. Adv Mater. 2003;15:1323–33. https://doi.org/10.1002/adma.200300010
Zhu Y, Yang B, Chen S, Du J. Polymer vesicles: mechanism, preparation, application, and responsive behavior. Prog Polym Sci. 2017;64:1–22. https://doi.org/10.1016/j.progpolymsci.2015.05.001
Tanner P, Baumann P, Enea R, Onaca O, Palivan C, Meier W. Polymeric vesicles: from drug carriers to nanoreactors and artificial organelles. Acc Chem Res. 2011;44:1039–49. https://doi.org/10.1021/ar200036k
Iqbal S, Blenner M, Alexander-Bryant A, Larsen J. Polymersomes for therapeutic delivery of protein and nucleic acid macromolecules: from design to therapeutic applications. Biomacromolecules. 2020;21:1327–50. https://doi.org/10.1021/acs.biomac.9b01754
Che H, van Hest JCM. Adaptive polymersome nanoreactors. ChemNanoMat. 2019;5:1092–109. https://doi.org/10.1002/cnma.201900245
Nishimura T, Akiyoshi K. Biotransporting biocatalytic reactors toward therapeutic nanofactories. Adv Sci. 2018;5:1800801. https://doi.org/10.1002/advs.201800801
Barenholz Y. Doxil® — The first FDA-approved nano-drug: Lessons learned. J Control Release. 2012;160:117–34. https://doi.org/10.1016/j.jconrel.2012.03.020
Boswell GW, Buell D, Bekersky I. AmBisome (Liposomal Amphotericin B): A Comparative Review. J Clin Pharmacol. 1998;38:583–92. https://doi.org/10.1002/j.1552-4604.1998.tb04464.x
Ichikawa K, Takeuchi Y, Yonezawa S, Hikita T, Kurohane K, Namba Y, Oku N. Antiangiogenic photodynamic therapy (PDT) using Visudyne causes effective suppression of tumor growth. Cancer Lett. 2004;205:39–48. https://doi.org/10.1016/j.canlet.2003.10.001
Wolfram J, Ferrari M. Clinical cancer nanomedicine. Nano Today. 2019;25:85–98. https://doi.org/10.1016/j.nantod.2019.02.005
Nishimura T, Shishi S, Sasaki Y, Akiyoshi K. Thermoresponsive polysaccharide graft polymer vesicles with tunable size and structural memory. J Am Chem Soc. 2020;142:11784–90. https://doi.org/10.1021/jacs.0c02290
Zhou Y, Yan D, Dong W, Tian Y. Temperature-responsive phase transition of polymer vesicles: real-time morphology observation and molecular mechanism. J Phys Chem B. 2007;111:1262–70. https://doi.org/10.1021/jp0673563
Qin S, Geng Y, Discher DE, Yang S. Temperature-controlled assembly and release from polymer vesicles of Poly(ethylene oxide)-block- poly(N-isopropylacrylamide). Adv Mater. 2006;18:2905–9. https://doi.org/10.1002/adma.200601019
Li Y, Lokitz BS, McCormick CL. Thermally responsive vesicles and their structural “locking” through polyelectrolyte complex formation. Angew Chem Int Ed. 2006;45:5792–5. https://doi.org/10.1002/anie.200602168
Cabane E, Malinova V, Menon S, Palivan CG, Meier W. Photoresponsive polymersomes as smart, triggerable nanocarriers. Soft Matter. 2011;7:9167–76. https://doi.org/10.1039/C1SM05880K
Zhao P, Xia J, Cao M, Xu H. Wavelength-controlled light-responsive polymer vesicle based on Se–S dynamic chemistry. ACS Macro Lett. 2020;9:163–8. https://doi.org/10.1021/acsmacrolett.9b00983
Wang X, Yao C, Zhang G, Liu S. Regulating vesicle bilayer permeability and selectivity via stimuli-triggered polymersome-to-PICsome transition. Nat Commun. 2020;11:1524. https://doi.org/10.1038/s41467-020-15304-x
Shi Z, Zhou Y, Yan D. Facile fabrication of pH-responsive and size-controllable polymer vesicles from a commercially available hyperbranched polyester. Macromol Rapid Commun. 2008;29:412–8. https://doi.org/10.1002/marc.200700673
Yu S, Azzam T, Rouiller I, Eisenberg A. “Breathing” vesicles. J Am Chem Soc. 2009;131:10557–66. https://doi.org/10.1021/ja902869q
Yan Q, Wang J, Yin Y, Yuan J. Breathing polymersomes: CO2-tuning membrane permeability for size-selective release, separation, and reaction. Angew Chem Int Ed. 2013;52:5070–3. https://doi.org/10.1002/anie.201300397
Ren T, Wu W, Jia M, Dong H, Li Y, Ou Z. Reduction-cleavable polymeric vesicles with efficient glutathione-mediated drug release behavior for reversing drug resistance. ACS Appl Mater Interfaces. 2013;5:10721–30. https://doi.org/10.1021/am402860v
Onaca O, Enea R, Hughes DW, Meier W. Stimuli-responsive polymersomes as nanocarriers for drug and gene delivery. Macromol Biosci. 2009;9:129–39. https://doi.org/10.1002/mabi.200800248
Li M-H, Keller P. Stimuli-responsive polymer vesicles. Soft Matter. 2009;5:927–37. https://doi.org/10.1039/B815725A
Feng A, Yuan J. Smart nanocontainers: progress on novel stimuli-responsive polymer vesicles. Macromol Rapid Commun. 2014;35:767–79. https://doi.org/10.1002/marc.201300866
Bordat A, Boissenot T, Nicolas J, Tsapis N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv Drug Deliv Rev. 2019;138:167–92. https://doi.org/10.1016/j.addr.2018.10.005
Roy D, Brooks WLA, Sumerlin BS. New directions in thermoresponsive polymers. Chem Soc Rev. 2013;42:7214–43. https://doi.org/10.1039/C3CS35499G
Nishimura T, Sasaki Y, Akiyoshi K. Biotransporting self-assembled nanofactories using polymer vesicles with molecular permeability for enzyme prodrug cancer therapy. Adv Mater. 2017;29:1702406. https://doi.org/10.1002/adma.201702406
Nishimura T, de Campo L, Iwase H, Akiyoshi K. Determining the hydration in the hydrophobic layer of permeable polymer vesicles by neutron scattering. Macromolecules. 2020;53:7546–51. https://doi.org/10.1021/acs.macromol.0c01261
Weiss TM, Narayanan T, Gradzielski M. Dynamics of spontaneous vesicle formation in fluorocarbon and hydrocarbon surfactant mixtures. Langmuir. 2008;24:3759–66. https://doi.org/10.1021/la703515j
Weiss TM, Narayanan T, Wolf C, Gradzielski M, Panine P, Finet S, Helsby WI. Dynamics of the self-assembly of unilamellar vesicles. Phys Rev Lett. 2005;94:038303. https://doi.org/10.1103/PhysRevLett.94.038303
Fuhrmans M, Müller M. Mechanisms of vesicle spreading on surfaces: coarse-grained simulations. Langmuir. 2013;29:4335–49. https://doi.org/10.1021/la400119e
Nguyen XD, Park DH, Paik H-j, Jeon HJ, Huh J, Go JS. Microfluidic tracking of the growth of polymeric vesicles in hydrodynamic flow. ACS Appl Polym Mater. 2020;2:5845–50. https://doi.org/10.1021/acsapm.0c01089
Yamada NL. Kinetic process of formation and reconstruction of small unilamellar vesicles consisting of long- and short-chain lipids. Langmuir. 2012;28:17381–8. https://doi.org/10.1021/la3026842
Battaglia G, Ryan AJ. Pathways of polymeric vesicle formation. J Phys Chem B. 2006;110:10272–9. https://doi.org/10.1021/jp060728n
Mable CJ, Derry MJ, Thompson KL, Fielding LA, Mykhaylyk OO, Armes SP. Time-resolved SAXS studies of the kinetics of thermally triggered release of encapsulated silica nanoparticles from block copolymer vesicles. Macromolecules. 2017;50:4465–73. https://doi.org/10.1021/acs.macromol.7b00475
Acknowledgements
This work was supported by the JSPS in the form of Grants-in-Aid for Scientific Research (S: 16H06313; B: 18H01845), the Asahi Glass Foundation, and the MEXT Leading Initiative for Excellent Young Researchers. SAXS experiments were conducted at the BL40B2 beamline of SPring-8 under proposal numbers 2017A1241 and 2020A1070.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of this manuscript. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nishimura, T., Sasaki, Y. & Akiyoshi, K. Thermoresponsive glycopolymer vesicles: in situ observation of morphological changes and triggered cargo release. Polym J 53, 1251–1258 (2021). https://doi.org/10.1038/s41428-021-00488-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00488-w