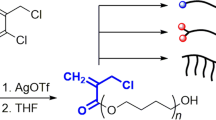
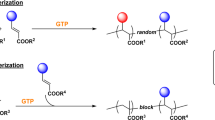
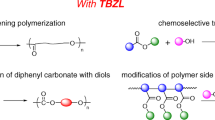
Abstract
The nucleophilic conjugate substitution of α-(substituted methyl)acrylate is a very convenient reaction that occurs at ambient temperature with a variety of nucleophiles, such as amines, thiols, phenols, enols, and carboxylic acids. The reaction is quantitative when the nucleophile and leaving group have distinctly different acidities, whereas it becomes dynamic and reversible if their acidities are similar. This review describes the fundamentals and applications of conjugate substitution reactions in polymer chemistry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kohsaka Y, Yamaguchi E, Kitayama T. Anionic alternating copolymerization of α-arylacrylates with methyl methacrylate: effect of monomer sequence on fluorescence. J Polym Sci Part A-Polym Chem. 2014;52:2806–14.
Yamada B, Kobatake S. Radical polymerization, co-polymerization, and chain transfer of α-substituted acrylic esters. Prog Polym Sci. 1994;19:1089–152.
Kodaira T, Liu QQ, Urushisaki M. Cyclopolymerization 24. Cyclopolymerizability of an unconjugated triene with functional groups with no homopolymerization tendency: radical polymerizations of N,N-diallyl-2-(methoxycarbonyl)allylamine. Macromol Chem Phys. 1997;198:3089–104.
Habaue S, Uno T, Okamoto Y. Stereospecific anionic polymerization of ethyl α-(1-pyrrolidinylmethyl)acrylate. Macromolecules. 1997;30:3125–6.
Yamada B, Konosu O. Control of branched structure of radical polymer by addition-fragmentation chain transfer - preparation of star polymer. Kobunshi Ronbunshu. 1997;54:723–30.
Onen A, Yagci Y. The effect of the heteroatom moiety of allylic salts on the addition fragmentation initiation of cationic polymerization. Macromole Chem Phys. 2001;202:1950–4.
Yilmaz F, Sudo A, Endo T. Allyl sulfonium salt as a novel initiator for active cationic polymerization of epoxide by shooting with radicals species. J Polym Sci Part A-Polym Chem. 2010;48:4178–83.
Kashman Y, Fishelson L, Ne’eman I. N-acyl-2-methylene-β-alanine methyl esters from the sponge Fasciospongia cavernosa. Tetrahedron. 1973;29:3655–7.
Yunker MB, Scheuer PJ. α-oxygenated fatty acids occurring as amides of 2-methylene-β-alanine in a marine sponge. Tetrahed Lett. 1978;19:4651–2.
Holm A, Scheuer PJ. Synthesis of α-methylene-β-alanine and one of its naturally occurring α-ketomides. Tetrahed Lett. 1980;21:1125–8.
Kohsaka Y, Matsumoto Y, Kitayama T. α-(Aminomethyl)acrylate: polymerization and spontaneous post-polymerization modification of beta-amino acid ester for a pH/temperature-responsive material. Polym Chem. 2015;6:5026–9.
Kohsaka Y, Kitaura T, Kitayama T. Precise synthesis of stereoregular polymethacrylates with end-functionality. Kobunshi Ronbunshu. 2015;72:385–94.
Kitaura T, Kitayama T. Anionic polymerization of methyl methacrylate with the aid of Lithium trimethylsilanolate (Me3SiOLi) - superior control of isotacticity and molecular weight. Macromol Rapid Commun. 2007;28:1889–93.
Nishiura T, Abe Y, Kitayama T. Syndiotactic-specific polymerization of methyl methacrylate with tert-butyllithium/trialkylaluminum in dichloromethane. Polym Bull. 2011;66:917–23.
Kitayama T, Nakagawa O, Kishiro S, Nishiura T, Hatada K. Control of main-chain stereostructure of graft polymers via stereospecific anionic copolymerization of syndiotactic poly(methyl methacrylate) macromonomer having methacryloyl function with methacrylate monomers. Polym J. 1993;25:707–20.
Usuki N, Satoh K, Kamigaito M. Synthesis of isotactic-block-syndiotactic poly(methyl methacrylate) via stereospecific living anionic polymerizations in combination with metal-halogen exchange, halogenation, and click reactions. Polymers. 2017;9:723.
Usuki N, Satoh K, Kamigaito M. Synthesis of Syndiotactic macrocyclic poly(methyl methacrylate) via transformation of the growing terminal in stereospecific anionic polymerization. Macromol Chem Phys. 2017;218:1700041.
Usuki N, Okura H, Satoh K, Kamigaito M. Synthesis and stereocomplexation of pmma-based star polymers prepared by a combination of stereospecific anionic polymerization and crosslinking radical polymerization. J Polym Sci Part A-Polym Chem. 2018;56:1123–7.
Kohsaka Y, Kurata T, Kitayama T. End-functional stereoregular poly(methyl methacrylate) with clickable C=C bonds: facile synthesis and thiol-ene reaction. Polym Chem. 2013;4:5043–7.
Kohsaka Y, KurataT, Yamamoto K, Ishihara S, Kitayama T. Synthesis and post-polymerization reaction of end-clickable stereoregular polymethacrylates via termination of stereospecific living anionic polymerization. Polym Chem. 2015;6:1078–108.
Lowe AB. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym Chem. 2010;1:17–36.
Lowe AB. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis: a first update. Polym Chem. 2014;5:4820–70.
Kohsaka Y, Yamamoto K, Kitayama T. Stereoregular poly(methyl methacrylate) with double-clickable omega-end: synthesis and click reaction. Polym Chem. 2015;6:3601–7.
Kohsaka Y, Ishihara S, Kitayama T. Termination of living anionic polymerization of butyl acrylate with alpha-(chloromethyl) acrylate for end-functionalization and application to the evaluation of monomer reactivity. Macromol Chem Phys. 2015;216:1534–9.
Okamoto Y, Habaue S, Uno T, Baraki H. Stereospecific polymerization of alpha-substituted acrylates. Macromol Symp. 2000;157:209–16.
Habaue S, Yamada H, Uno T, Okamoto Y. Stereospecific polymerization of benzyl α-(alkoxymethyl) acrylates. J Polym Sci Part A-Polym Chem. 1997;35:721–6.
Uno T, Habaue S, Okamoto Y. Stereospecific polymerization of alpha-(menthoxymethyl)acrylate. Enantiomer. 2000;5:29–36.
Kohsaka Y, Yamamoto K, Suzawa K, Kitayama T. Synthesis of isotactic poly[α-(hydroxymethyl)acrylate] by anionic polymerization of the protected monomer. Polym Bull. 2017;74:1935–48.
Kohsaka Y, Matsumoto., Zhang TY, Matsuhashi Y, Kitayama T. α-Exomethylene lactone possessing acetal-ester linkage: polymerization and postpolymerization modification for water-soluble polymer. J Polym Sci Part A-Polym Chem. 2016;54:955–61.
Vargas JS, Zilliox JG, Rempp P, Franta E. Cationic synthesis of macromers. Polym Bull. 1980;3:83–89.
Kohsaka Y, Koyama Y, Takata T. Graft polyrotaxanes: a new class of graft copolymers with mobile graft chains. Angew Chem Int Ed. 2011;50:10417–20.
Kohsaka Y, Nagatsuka N. End-reactive poly(tetrahydrofuran) for functionalization and graft copolymer synthesis via a conjugate substitution reaction. Polym J. 2020;52:75–81.
Burgess FJ, Cunliffe AV, Richards DH, Thompson D. Organic halides as cationic initiators. Polymer. 1978;19:334–40.
Kohsaka Y, Hagiwara K, Ito K. Polymerization of α-(halomethyl)acrylates through sequential nucleophilic attack of dithiols using a combination of addition-elimination and click reactions. Polym Chem. 2017;8:976–9.
Kohsaka Y, Miyazaki T, Hagiwara K. Conjugate substitution and addition of alpha-substituted acrylate: a highly efficient, facile, convenient, and versatile approach to fabricate degradable polymers by dynamic covalent chemistry. Polym Chem. 2018;9:1610–7.
Mathias LJ, Dickerson CW. Acrylate-containing oligo(ether ester) cross-linking agents with controlled molecular-weights via end-group termination. Macromolecules. 1991;24:2048–53.
Mathias LJ, Kusefoglu SH, Kress AO, Lee S, Wright JR, Culberson DA, Warren SC, Warren RM, Huang S, Lopez DR, Ingram JE, Dickerson CW, Jeno M, Halley RJ, Colletti RF, Cei G, Geiger CC. Multifunctional acrylate monomers, dimers and oligomers - applications from contact-lenses to wood-polymer composites. Makromol Chem-Macrom Symp. 1991;51:153–67.
Ji SH, Bruchmann B, Klok HA. Exploring the scope of the baylis-hillman reaction for the synthesis of side-chain functional polyesters. Macromol Chem Phys. 2011;212:2612–8.
Ji SH, Bruchmann B, Klok HA. Synthesis of side-chain functional polyesters via baylis-hillman polymerization. Macromolecules. 2011;44:5218–26.
Robert T, Friebel S. Itaconic acid - a versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016;18:2922–34.
Tang XY, Hong M, Falivene L, Caporaso L, Cavallo L, Chen E-YX. The quest for converting biorenewable bifunctional alpha-methylene-gamma-butyrolactone into degradable and recyclable polyester: controlling vinyl-addition/ring-opening/cross-linking pathways. J Am Chem Soc. 2016;138:14326–37.
Hong M, Chen E-YX. Coordination ring-opening copolymerization of naturally renewable alpha-methylene-gamma-butyrolactone into unsaturated polyesters. Macromolecules. 2014;47:3614–24.
Kohsaka Y, Hiramatsu A. Synthesis and properties of polyethers containing 1,3-butadiene skeleton in the backbone. Chem Lett. 2019;48:894–7.
Nicolaou KC, Montagnon T, Snyder SA. Tandem reactions, cascade sequences, and biomimetic strategies in total synthesis. Chem Commun. 2003;39:551–64.
Tietze LF. Domino reactions in organic synthesis. Chem Rev. 1996;96:115–36.
Kakuchi R. Multicomponent reactions in polymer synthesis. Angew Chem Int Ed. 2014;53:46–48.
Kakuchi R. The dawn of polymer chemistry based on multicomponent reactions. Polym J. 2019;51:945–53.
Koyama Y, Gudeangadi PG. One-pot synthesis of alternating peptides exploiting a new polymerization technique based on Ugi’s 4CC reaction. Chem Commun. 2017;53:3846–9.
Xu YC, Ren WM, Zhou H, Gu GG, Lu XB. Functionalized polyesters with tunable degradability prepared by controlled ring-opening (co)polymerization of lactones. Macromolecules. 2017;50:3131–42.
Xu YC, Zhou H, Sun XY, Ren WM, Lu XB. Crystalline polyesters from CO2 and 2-butyne via alpha-methylene-beta-butyrolactone intermediate. Macromolecules. 2016;49:5782–7.
Kohsaka Y, Yamashita M, Matsuhashi Y, Yamashita S. Synthesis of poly(conjugated ester)s by ring-opening polymerization of cyclic hemiacetal ester bearing acryl skeleton. Eur Polym J. 2019;120:109185.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kohsaka, Y. Conjugate substitution reaction of α-(substituted methyl)acrylates in polymer chemistry. Polym J 52, 1175–1183 (2020). https://doi.org/10.1038/s41428-020-0376-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0376-z