Abstract
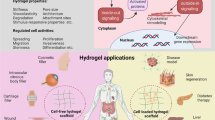
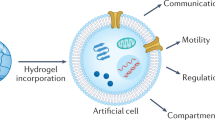
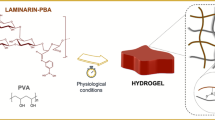
Biofunctional hydrogels prepared by a peroxidase, especially horseradish peroxidase (HRP), serve as an excellent class of materials or platform for the development of cellular scaffolds because their biocompatibility and mild and tunable reaction conditions provide them with desirable properties. In this focus review, we summarize our decade of research into HRP-mediated fabrication of biofunctional hydrogels and their applications, in particular cell culture scaffolds. A brief overview of potential substrates employed in HRP and improvement of the HRP hydrogelation system from the initial step until the hydrogen peroxide removal stage in an effort to meet environmental standards is discussed. We highlight our system and describe its biocompatibility and ability to functionalize molecules to support biofabrication by increasing cellular adhesiveness, retaining growth factor affinity, and finally accelerating the formation of two- and three-dimensional multicellular architectures. In the last section, we outline the adoption of hydrogelation as a self-standing, compartmentalized reaction system, i.e., the use of hydrogel marble to conduct cell-free biosynthesis. We believe that this HRP-mediated hydrogel system offers great potential not only as a cell culture scaffold but also for various biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Green JJ, Elisseeff JH. Mimicking biological functionality with polymers for biomedical applications. Nature. 2016;540:386–94.
Lee SC, Kwon IK, Park K. Hydrogels for delivery of bioactive agents: a historical perspective. Adv Drug Deliv Rev. 2013;65:17–20.
Kopeček J. Swell gels. Nature. 2002;417:389–91.
Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12.
Kang DH, Kim D, Wang S, Song D, Yoon MH. Water-insoluble, nanocrystalline, and hydrogel fibrillar scaffolds for biomedical applications. Polym J. 2018;50:637–47.
Schneider-Barthold C, Baganz S, Wilhelmi M, Scheper T, Pepelanova I. Hydrogels based on collagen and fibrin - frontiers and applications. BioNanoMaterials. 2016;17:3–12.
Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6:1–10.
Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–90.
Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods. 2016;13:405–14.
Spicer CD. Hydrogel scaffolds for tissue engineering: the importance of polymer choice. Polym Chem. 2020;11:184–219.
Buwalda SJ, Boere KWM, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE. Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release. 2014;190:254–73.
Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–21.
Choi JR, Yong KW, Choi JY, Cowie AC. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques. 2019;66:40–53.
Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R. Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules. 2007;8:1844–50.
Hu BH, Messersmith PB. Rational design of transglutaminase substrate peptides for rapid enzymatic formation of hydrogels. J Am Chem Soc. 2003;125:14298–9.
Mironi-Harpaz I, Wang DY, Venkatraman S, Seliktar D. Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012;8:1838–48.
Steinhilber D, Haag R. Multifunctional dendritic polyglycerol nano- and microgels for encapsulation and release of functional. Biomacromolecules. 2011;20:7545.
Moreira Teixeira LS, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials. 2012;33:1281–90.
Buwalda SJ, Vermonden T, Hennink WE. Hydrogels for therapeutic delivery: current developments and future directions. Biomacromolecules. 2017;18:316–30.
Kurisawa M, Chung JE, Yang YY, Gao SJ, Uyama H. Injectable biodegradable composed of hyaluronic acid-conjugates for drug delivery and tissue engineering. Chem Chomm. 2005;34:4312–4.
Kim EH, Lim S, Kim TE, Jeon IO, Choi YS. Preparation of in situ injectable chitosan/gelatin hydrogel using an acid-tolerant tyrosinase. Biotechnol Bioprocess Eng. 2018;23:500–6.
Huber D, Grzelak A, Baumann M, Borth N, Schleining G, Nyanhongo GS, et al. Anti-inflammatory and anti-oxidant properties of laccase-synthesized phenolic-O-carboxymethyl chitosan hydrogels. N. Biotechnol. 2018;40:236–44.
Huber D, Tegl G, Baumann M, Sommer E, Gorji EG, Borth N, et al. Chitosan hydrogel formation using laccase activated phenolics as cross-linkers. Carbohydr Polym 2017;157:814–22.
Yung CW, Bentley WE, Barbari TA. Diffusion of interleukin-2 from cells overlaid with cytocompatible enzyme-crosslinked gelatin hydrogels. J Biomed Mater Res Part A. 2010;95:25–32.
Cambria E, Renggli K, Ahrens CC, Cook CD, Kroll C, Krueger AT, et al. Covalent modification of synthetic hydrogels with bioactive proteins via sortase-mediated ligation. Biomacromolecules. 2015;16:2316–26.
Yang Z, Gu H, Fu D, Gao P, Lam JK, Xu B. Enzymatic formation of supramolecular hydrogels. Adv Mater. 2004;16:1440–4.
Bakota EL, Aulisa L, Galler KM, Hartgerink JD. Enzymatic cross-linking of a nanofibrous peptide hydrogel. Biomacromolecules. 2011;12:82–7.
Mosiewicz KA, Johnsson K, Lutolf MP. Phosphopantetheinyl transferase-catalyzed formation of bioactive hydrogels for tissue engineering. J Am Chem Soc. 2010;132:5972–4.
Yang Z, Ho PL, Liang G, Chow KH, Wang Q, Cao Y, et al. Using β-lactamase to trigger supramolecular hydrogelation. J Am Chem Soc. 2007;129:266–7.
Toledano S, Williams RJ, Jayawarna V, Ulijn RV. Enzyme-triggered self-assembly of peptide hydrogels via reversed hydrolysis. J Am Chem Soc. 2006;128:1070–1.
Xie Y, Huang R, Qi W, Wang Y, Su R, He Z. Enzyme-substrate interactions promote the self-assembly of amino acid derivatives into supramolecular hydrogels. J Mater Chem B. 2016;4:844–51.
Hai Z, Li J, Wu J, Xu J, Liang G. Alkaline phosphatase-triggered simultaneous hydrogelation and chemiluminescence. J Am Chem Soc. 2017;139:1041–4.
Yang Z, Liang G, Wang L, Xu B. Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramolecular hydrogel in vivo. J Am Chem Soc. 2006;128:3038–43.
Rowe SL, Lee SY, Stegemann JP. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007;3:59–67.
Sofia SJ, Singh A, Kaplan DL. Peroxidase-catalyzed crosslinking of functionalized polyaspartic acid polymers. J Macromol Sci - Pure Appl Chem. 2002;39 A:1151–81.
Lopes GR, Pinto DCGA, Silva AMS. Horseradish peroxidase (HRP) as a tool in green chemistry. RSC Adv. 2014;4:37244–65.
Sakai S, Nakahata M. Horseradish peroxidase catalyzed hydrogelation for biomedical, biopharmaceutical, and biofabrication applications. Chem Asian J. 2017;12:3098–109.
Bae JW, Choi JH, Lee Y, Park KD. Horseradish peroxidase-catalysed in situ -forming hydrogels for tissue-engineering applications. J Tissue Eng Regen Med. 2015;9:1225–32.
Guebitz GM, Nyanhongo GS. Enzymes as green catalysts and interactive biomolecules in wound dressing hydrogels. Trends Biotechnol. 2018;36:1040–53.
Lee F, Bae KH, Kurisawa M. Injectable hydrogel systems crosslinked by horseradish peroxidase. Biomed Mater. 2016;11:014101.
Shakya AK, Nandakumar KS. An update on smart biocatalysts for industrial and biomedical applications. J R Soc Interface. 2018;15:20180062.
Krainer FW, Glieder A. An updated view on horseradish peroxidases: recombinant production and biotechnological applications. Appl Microbiol Biotechnol. 2015;99:1611–25.
Veitch NC. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry. 2004;65:249–59.
Rodríguez-López JN, Lowe DJ, Hernández-Ruiz J, Hiner ANP, García-Cánovas F, Thorneley RNF. Mechanism of reaction of hydrogen peroxide with horseradish peroxidase: identification of intermediates in the catalytic cycle. J Am Chem Soc. 2001;123:11838–47.
Zakharova GS, Uporov IV, Tishkov VI. Horseradish peroxidase: modulation of properties by chemical modification of protein and heme. Biochem. 2011;76:1391–401.
Kobayashi S, Uyama H, Kimura S. Enzymatic polymerization. Chem Rev. 2001;101:3793–818.
Wang LS, Chung JE, Lee F. (12) Patent Application Publication (10) Pub. No.: US 2010/0074956 A1. 1, (2010).
Hoang Thi TT, Lee Y, Le Thi P, Park KD. Engineered horseradish peroxidase-catalyzed hydrogels with high tissue adhesiveness for biomedical applications. J Ind Eng Chem. 2019;78:34–52.
Raia NR, Partlow BP, McGill M, Kimmerling EP, Ghezzi CE, Kaplan DL. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials. 2017;131:58–67.
Kuo KC, Lin RZ, Tien HW, Wu PY, Li YC, Melero-Martin JM, et al. Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater 2015;27:151–66.
Sakai S, Yamada Y, Zenke T, Kawakami K. Novel chitosan derivative soluble at neutral pH and in-situ gellable via peroxidase-catalyzed enzymatic reaction. J Mater Chem. 2009;19:230–5.
Chen F, Yu S, Liu B, Ni Y, Yu C, Su Y, et al. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci Rep. 2016;6:20014.
Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials. 2009;30:3371–7.
Jin R, Hiemstra C, Zhong Z, Feijen J. Enzyme-mediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials. 2007;28:2791–800.
Zhou B, Wang P, Cui L, Yu Y, Deng C, Wang Q, et al. Self-crosslinking of silk fibroin using H2O2-horseradish peroxidase system and the characteristics of the resulting fibroin membranes. Appl Biochem Biotechnol. 2017;182:1548–63.
Sakai S, Kawakami K. Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta Biomater. 2007;3:495–501.
Park KM, Shin YM, Joung YK, Shin H, Park KD. In situ forming hydrogels based on tyramine conjugated 4-Arm-PPO-PEO via enzymatic oxidative reaction. Biomacromolecules. 2010;11:706–12.
Lee SH, Lee Y, Lee SW, Ji HY, Lee JH, Lee DS, et al. Enzyme-mediated cross-linking of Pluronic copolymer micelles for injectable and in situ forming hydrogels. Acta Biomater. 2011;7:1468–76.
Sakai S, Tsumura M, Inoue M, Koga Y, Fukano K, Taya M. Polyvinyl alcohol-based hydrogel dressing gellable on-wound via a co-enzymatic reaction triggered by glucose in the wound exudate. J Mater Chem B. 2013;1:5067–75.
Sun Y, Deng Z, Tian Y, Lin C. Horseradish peroxidase-mediated in situ forming hydrogels from degradable tyramine-based poly(amido amine)s. J Appl Polym Sci. 2013;127:40–8.
Macdougall LJ, Pérez-Madrigal MM, Arno MC, Dove AP. Nonswelling thiol-yne cross-linked hydrogel materials as cytocompatible soft tissue scaffolds. Biomacromolecules. 2018;19:1378–88.
Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci Part B Polym Phys. 2011;49:832–64.
Moriyama K, Wakabayashi R, Goto M, Kamiya N. Characterization of enzymatically gellable, phenolated linear poly(ethylene glycol) with different molecular weights for encapsulating living cells. Biochem Eng J. 2014;93:25–30.
Minamihata K, Goto M, Kamiya N. Protein heteroconjugation by the peroxidase-catalyzed tyrosine coupling reaction. Bioconjug Chem. 2011;22:2332–8.
Minamihata K, Goto M, Kamiya N. Site-specific protein cross-linking by peroxidase-catalyzed activation of a tyrosine-containing peptide tag. Bioconjug Chem. 2011;22:74–81.
Moriyama K, Minamihata K, Wakabayashi R, Goto M, Kamiya N. Enzymatic preparation of streptavidin-immobilized hydrogel using a phenolated linear poly(ethylene glycol). Biochem Eng J. 2013;76:37–42.
Ohya Y. Temperature-responsive biodegradable injectable polymer systems with conveniently controllable properties. Polym J. 2019;51:997–1005.
Kambe Y, Tokushige T, Mahara A, Iwasaki Y, Yamaoka T. Cardiac differentiation of induced pluripotent stem cells on elastin-like protein-based hydrogels presenting a single-cell adhesion sequence. Polym J. 2019;51:97–105.
Sakai S, Komatani K, Taya M. Glucose-triggered co-enzymatic hydrogelation of aqueous polymer solutions. RSC Adv. 2012;2:1502–7.
Singh S, Topuz F, Hahn K, Albrecht K, Groll J. Embedding of active proteins and living cells in redox-sensitive hydrogels and nanogels through enzymatic cross-linking. Angew Chem - Int Ed. 2013;52:3000–3.
Moriyama K, Minamihata K, Wakabayashi R, Goto M, Kamiya N. Enzymatic preparation of a redox-responsive hydrogel for encapsulating and releasing living cells. Chem Commun. 2014;50:5895–8.
Obinger C, Burner U, Ebermann R. Generation of hydrogen peroxide by plant peroxidases mediated thiol oxidation. Phyt Ann Rei Bot. 1997;37:219–26.
Dunford HB, Adeniran AJ. Hammett ϱσ correlation for reactions of horseradish peroxidase compound II with phenols. Arch Biochem Biophys. 1986;251:536–42.
Matsusaki M, Yoshida H, Akashi M. The construction of 3D-engineered tissues composed of cells and extracellular matrices by hydrogel template approach. Biomaterials. 2007;28:2729–37.
Moriyama K, Naito S, Wakabayashi R, Goto M, Kamiya N. Enzymatically prepared redox-responsive hydrogels as potent matrices for hepatocellular carcinoma cell spheroid formation. Biotechnol J. 2016;11:1452–60.
Moriyama K, Wakabayashi R, Goto M, Kamiya N. Enzyme-mediated preparation of hydrogels composed of poly(ethylene glycol) and gelatin as cell culture platforms. RSC Adv. 2015;5:3070–3.
Jones CJ, Beni S, Limtiaco JFK, Langeslay DJ, Larive CK. Heparin characterization: challenges and solutions. Annu Rev Anal Chem. 2011;4:439–65.
Tae G, Kim Y-J, Choi W-I, Kim M, Stayton PS, Hoffman AS. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8:1979–86.
Ramadhan W, Kagawa G, Hamada Y, Moriyama K, Wakabayashi R, Minamihata K, et al. Enzymatically prepared dual functionalized hydrogels with gelatin and heparin to facilitate cellular attachment and proliferation. ACS Appl Bio Mater. 2019;2:2600–9.
Claaßen C, Sewald L, Tovar G, Borchers K. Controlled release of vascular endothelial growth factor from heparin-functionalized gelatin Type A and albumin hydrogels. Gels. 2017;3:35.
Li Z, Qu T, Ding C, Ma C, Sun H, Li S, et al. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 2015;13:88–100.
Kamiya N, Ohama Y, Minamihata K, Wakabayashi R, Goto M. Liquid marbles as an easy-to-handle compartment for cell-free synthesis and in situ immobilization of recombinant. Proteins Biotechnol J. 2018;13:1–5.
Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI No. JP17K19016. The authors thank the Center of Advanced Instrumental Analysis, Kyushu University, and Nanotechnology Platform Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) for facility support as well as Dr Kosuke Minamihata for the discussion and assistance in protein production.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wakabayashi, R., Ramadhan, W., Moriyama, K. et al. Poly(ethylene glycol)-based biofunctional hydrogels mediated by peroxidase-catalyzed cross-linking reactions. Polym J 52, 899–911 (2020). https://doi.org/10.1038/s41428-020-0344-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0344-7