Abstract
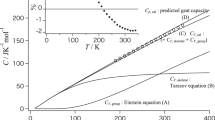
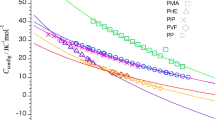
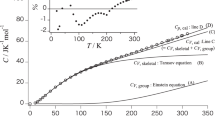
Despite being a useful variable for the quantitative analysis of the thermodynamic properties of polymers, the absolute value of heat capacity remains poorly understood for amorphous polymers. This study evaluates the absolute values of the heat capacities of amorphous polymers by using the Tarasov and Einstein equations to calculate the frequencies of the skeletal and group vibration modes, respectively. Moreover, the difference between the heat capacity measured at constant pressure and that obtained at constant volume is added as a correction factor when estimating the heat capacity. The heat capacity that contributes to skeletal vibrations can be expressed using the one- and three-dimensional Tarasov equations, and the contribution of group vibrations can be determined by substituting the absorption frequency obtained from infrared absorption measurements into the frequency value of the Einstein equation. Only three fitting parameters were used to reproduce the absolute value of the heat capacity obtained from the combination of these equations. We used this approach to reproduce the heat capacities of 16 main chain-type amorphous polymers having a carbon backbone. The reproduced and experimental heat capacities of all the samples below their glass transition temperature agreed within less than ±2.5%.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wagman DD, Evans WH, Parker VB, Schumm RH, Halow I, Bailey SM. The NBS tables of Chemical Thermodynamic Properties: selected values for inorganic and C1 and C2 organic substances in SI units. J Phys Chem Ref Dat. 1982;11:2.
Gopal ESR. Specific heats at low temperatures. London: Springer Science & Business Media; 2012.
Wunderlich B. Thermal analysis of polymeric materials. Heidelberg: Springer Science & Business Media; 2005.
Šesták J, Mareš JJ, Hubík P. Glassy, amorphous and nano-crystalline materials: thermal physics, analysis, structure and properties. Hot topics in thermal analysis and calorimetry 8. New York: Springer Science & Business Media; 2010.
Gibson GE, Giauque WF. The third law of thermodynamics. Evidence from the specific heats of glycerol that the entropy of a glass exceeds that of a crystal at the absolute zero. J Am Chem Soc. 1923;45:93–104.
Haida O, Matsuo T, Suga H, Seki S. Calorimetric study of the glassy state X. Enthalpy relaxation at the glass-transition temperature of hexagonal ice. J Chem Thermodyn. 1974;6:815–25.
Tajima Y, Matsuo T, Suga H. Calorimetric study of phase transition in hexagonal ice doped with alkali hydroxides. J Phys Chem Solids. 1984;45:1135–44.
Kume Y, Muraoka H, Yamamuro O, Matsuo T. Deuteration-induced phase transition in ammonium hexachloroplumbate. J Chem Phys. 1988;108:4090–7.
Miyazaki Y, Wang Q, Sato A, Saito K, Yamamoto M, Kitagawa H, et al. Heat capacity of the halogen-bridged mixed-valence complex Pt2 (dta)4I (dta = CH3CS2 −). J Phys Chem B. 2002;106:197–202.
Yamamura Y, Nakajima N, Tsuji T, Koyano M, Iwasa Y, Katayama S, et al. Low temperature heat capacities and Raman spectra of negative thermal expansion compounds ZrW2O8 and HfW2O8. Phys Rev B. 2002;66:014301.
Matsuo T, Maekawa T, Inaba A, Yamamuro O, Ohama M, Ichikawa M, et al. Isotope-dependent crystalline phases at ambient temperature: spectroscopic and calorimetric evidence for a deuteration-induced phase transition at 320 K in α-DCrO2. J Mol Struct. 2006;790:129–34.
Saito K, Sato A, Kikuchi K, Nishikawa H, Ikemoto I, Sorai M. Calorimetric study of metal-insulator transition in (DIMET) 2I 3. J Phys Soc Jpn. 2000;69:3602–6.
Yamamuro O, Tsukushi I, Lindqvist A, Takahara S, Ishikawa M, Matsuo T. Calorimetric study of glassy and liquid toluene and ethylbenzene: thermodynamic approach to spatial heterogeneity in glass-forming molecular liquids. J Phys Chem B. 1998;102:1605–9.
Pyda M, Bartkowiak M, Wunderlich B. Computation of heat capacities of solids using a general Tarasov equation. J Therm Anal. 1998;52:631–56.
Einstein A. Die Plancksche theorie der strahlung und die theorie der spezifischen wärme. Ann der Phys. 1907;327:180–90.
Debye P. Zur theorie der spezifischen wärmen. Ann der Phys. 1912;344:789–839.
Nernst W, Lindemann FA. Spezifische wärme und quantentheorie. Z Elektrochem. 1911;17:817–27.
Tarasov VV, Yunitskii GA. Theory of heat capacity of chain and layer structures. Russ J Phys Chem. 1965;39:1109–11.
Jianye W. Heat capacities of polymers in physical properties of polymers handbook. In: James E. Mark, editor. New York: Springer; 2007. p. 145–54.
Domalski ES, Hearing ED. Heat capacities and entropies of organic compounds in the condensed phase. Volume III. J Phys Chem Ref Data. 1996;25:1–525.
Wunderlich B. The ATHAS database on heat capacities of polymers. Pure Appl Chem. 1995;67:1019–26.
Chang SS, Bestul AB. Heat capacities of cis-1, 4-Polyisoprene from 2 to 360 K. J Res Natl Bur Stand A Phys Chem. 1971;75A:113–20.
Dainton FS, Evans DM, Hoare FE, Melia TP. Thermodynamic functions of linear high polymers: part I—polyoxymethylene. Polymer. 1962;3:263–321.
Bourdariat J, Berton A, Chaussy J, Isnard R, Odin J. Influence of cooling rate on the heat capacity and thermal transitions of amorphous polyhexene-1. Polymer. 1973;14:167–70.
Furukawa GT, Reilly ML. Heat capacity of polyisobutylene from 0 to 380 K. J Res Natl Bur Stand. 1956;56:285.
Chang SS. Heat capacity and thermodynamic properties of poly (vinyl chloride). J Res Natl Bur Stand. 1977;82:9–17.
Lebedev BV, Rabinovich IB, Busarina VA. Heat capacity of vinyl chloride, polyvinylchloride and polyvinylidene chloride in the region of 60–300°K. Polym Sci USSR. 1967;9:545–52.
Lee WK, Choy CL. Heat capacity of fluoropolymers. J Polym Sci. 1975;13:619–35.
Furukawa GT, McCoskey RE, King GJ. Calorimetric properties of polytetrafluoroethylene (Teflon) from 0 to 365 K. J Res Natl Bur Stand. 1952;49:273–8.
Lebedev BV, Rabinovich IB, Martynenko LYa. The heat capacity and thermodynamic functions of acrylonitrile and polyacrylonitrile. Polym Sci USSR. 1967;9:1841–9.
Rabinovich IB, Lebedev BV. Thermodynamics of vinyl monomers and polymers. V. Measurement of heat capacity and a calculation of the thermodynamic functions of poly (methyl acrylate), poly-(methyl methacrylate), poly (methacrylamide), poly (α-methylstyrene) and poly (vinyl alcohol). Tr Khim Tekhnol. 1967;2:36.
Pavlinov LI, Rabinovich IB, Okladnov NA, Arzhakov SA. Heat capacity of copolymers of methyl methacrylate with methacrylic acid in the region 25–190 °C. Polym Sci USSR. 1967;9:539–44.
NIST Chemistry WebBook SRD69. 100 Bureau Drive Gaithersburg, MD 20899. 1901. https://webbook.nist.gov/cgi/cbook.cgi?ID=C106989&Units=SI&Mask=80#IR-Spec. Accessed 7 Nov 2019.
Stromberg RR, Straus S, Achhammer BG. Infrared spectra of thermally degraded poly (vinyl chloride). J Res Natl Bur Stand. 1958;60:147–52.
Krimm S. Infrared spectra of high polymers. Fortschr Hochpolym Forsch Bd. 1960;2:51–172.
Nallasamy P, Mohan S. Vibrational spectra of cis-1, 4-polyisoprene. Arab J Sci Eng. 2004;29:17–26.
NIST Chemistry WebBook SRD69. 100 Bureau Drive Gaithersburg, MD 20899. 1901. https://webbook.nist.gov/cgi/cbook.cgi?ID=C592416&Type=IR-SPEC&Index=1#IR-SPEC. Accessed 7 Nov 2019.
Adams MA, Gabrys BJ, Zajac WM, Peiffer DG. High-resolution incoherent inelastic neutron scattering spectra of polyisobutylene and polyisoprene. Macromolecules. 2005;38:160–6.
Hong JW, Lando JB, Koenig JL, Chough SH, Krimm S. Normal-mode analysis of infrared and Raman spectra of poly (vinyl fluoride). Vib Spectros. 1992;3:55–66.
Nallasamy P, Mohan S. Vibrational spectroscopic characterization of form II poly (vinylidene fluoride). Indian J Pure Ap Phys. 2005;43:821–7.
Adams CJ, Downs AJ. The vibrational spectra and structures of selenium tetrafluoride and tellurium tetrafluoride: including a matrix-isolation study. Spectrochim Acta A. 1972;28:1841–54.
Liang CY, Krimm S. Infrared spectra of high polymers. VII. Polyacrylonitrile. J Polym Sci. 1958;31:513–22.
Willis HA, Zichy VJI, Hendra PJ. The laser-Raman and infra-red spectra of poly (methyl methacrylate). Polymer. 1969;10:737–46.
Wei-Fang S. Principles of polymer design and synthesis. Wei-Fang Su, editor. Berlin Heidelberg: Springer-Verlag; 2013. 53.
Bares V, Wunderlich B. Heat capacity of molten polymers. J Polym Sci. 1973;11:861–73.
Alger M. Polymer science dictionary. London: Springer Science & Business Media; 1996;463.
PolymerProcessing.com. 66 Buckskin Drive Weston, MA 02493 USA. 2001. http://www.polymerprocessing.com/index.html. Accessed 7 Nov 2019.
Yagi T. Heat of fusion and crystallization kinetics of Poly(trifluoroethylene). Polym J. 1980;12:9–15.
Wunderlich B, Gaur U. Differential scanning calorimetry of flexible, linear macromolecules. ACS Polymer preprints. American Chemical Society. Div Polym Chem. 1981;22:308.
Pyda M, Nowak-Pyda E, Mays J, Wunderlich B. Heat capacity of poly (butylene terephthalate). J Polym Sci. 2004;42:4401–11.
Kauzmann K. The nature of the glassy state and the behavior of liquids at low temperatures. Chem Rev. 1948;43:219–56.
Angell CA, Choi Y. Crystallization and vitrification in aqueous systems. J Microsc. 1986;141:251–61.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for reviewing the English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yokota, M., Sugane, K., Tsukushi, I. et al. Evaluation of the heat capacity of amorphous polymers composed of a carbon backbone below their glass transition temperature. Polym J 52, 765–774 (2020). https://doi.org/10.1038/s41428-020-0317-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0317-x
This article is cited by
-
Analysis of the configurational heat capacity of polystyrene and its monomer and oligomer above the glass transition temperature
Polymer Journal (2022)
-
Configurational heat capacity of various polymers above the glass transition temperature
Polymer Journal (2022)
-
Estimation of the configurational heat capacity of polyisobutylene, isobutane and 2,2,4-isomethylpentane above the glass transition temperature
Polymer Journal (2021)
-
Prediction of the heat capacity of main-chain-type polymers below the glass transition temperature
Polymer Journal (2020)
-
Heat capacities of polymer solids composed of polyesters and poly(oxide)s, evaluated below the glass transition temperature
Polymer Journal (2020)