Abstract
Direct CO2 capture from the air, so-called direct air capture (DAC), has become inevitable to reduce the concentration of CO2 in the atmosphere. Current DAC technologies consider only sorbent-based systems. Recently, there have been reports that show ultrahigh CO2 permeances in gas separation membranes and thus membrane separation could be a potential new technology for DAC in addition to sorbent-based CO2 capture. The simulation of chemical processes has been well established and is commonly used for the development and performance assessment of industrial chemical processes. These simulations offer a credible assessment of the feasibility of membrane-based DAC (m-DAC). In this paper, we discuss the potential of m-DAC considering the state-of-the-art performance of organic polymer membranes. The multistage membrane separation process was employed in process simulation to estimate the energy requirements for m-DAC. Based on the analysis, we propose the target membrane separation performance required for m-DAC with competitive energy expenses. Finally, we discuss the direction of future membrane development for DAC.
Similar content being viewed by others
Introduction
Carbon dioxide emissions into the atmosphere are the main reason for climate change. Drastic reductions in CO2 emissions into the atmosphere are unavoidable to meet the 1.5 °C scenario recommended by the Intergovernmental Panel on Climate Change [1]. Unfortunately, it has become apparent that the 1.5 °C scenario cannot be achieved simply by reducing CO2 emissions to the atmosphere [2,3,4]. This has led to the need for a more progressive approach, called negative emission technologies. These technologies include direct CO2 capture (direct air capture (DAC)) from ambient air by chem- or physisorption processes and other approaches, such as the use of bioderived energy combined with carbon capture and storage (BECCS), enhanced weathering of minerals, afforestation, and reforestation [5,6,7,8]. Among these technologies, DAC has a removal capacity comparable to that of BECCS (~3.3 Gt-C/year), which requires a large amount of land and water [9,10,11]. While the potential of DAC based on sorption technologies has been proven, it requires considerable energy to desorb CO2 from the sorbents [12, 13].
Membrane separation is also one of the major technologies for capturing CO2. Compared to other conventional capture methods, CO2 capture by permselective membranes has advantages because of its smaller footprint and simpler setup and operation. However, large-scale membrane separation is still in its infancy, with only a few pilot plants operating at CO2 mass emission points, such as coal-fired power plants that emit flue gases containing 10–15% CO2 [14]. Merkel et al. concluded that improved gas permeance in membranes is more critical for reducing capture cost than enhanced selectivity [15]. Compared to concentrated CO2 emission sources, the partial pressure of CO2 in the air is only 40 Pa, which is considered too small to pass CO2 through membranes effectively. Keith et al. also mentioned that DAC by membranes seems implausible, given the relatively low molecular fluxes through membranes [16]. Based on these considerations, membrane-based DAC (m-DAC) has never been considered [6].
Recently, many efforts have been devoted to the development of polymeric materials with high CO2 permeance. For example, Yoo et al. reported defect-free Teflon-based membranes possessing a CO2 permeance of ~31,500 GPU (GPU: gas permeance unit, 1 GPU = 7.5 × 10−12 m3(STP) m−2 s−1 Pa−1, STP: standard temperature and pressure) with a CO2/N2 selectivity of 3.3 [17]. We have also reported free-standing siloxane nanomembranes with high CO2 permeances exceeding 40,000 GPU [18]. Such membranes with ultrahigh gas permeance and selectivity would have the potential to capture CO2 efficiently, even from the air, since the gas permeance resistance through the membranes is quite small. Based on recent achievements in the membrane field, it is meaningful to evaluate the potential of membrane separation as an alternative DAC process. Analysis of membrane separation using process simulation has been well established based on chemical engineering aspects. This process analysis based on computational chemical engineering can assess the performance and feasibility of m-DAC from a realistic standpoint.
In this paper, we explored the potential of m-DAC based on process simulation with consideration of the state-of-the-art CO2 separation membrane performance. The future direction of membrane development for DAC is discussed.
Preliminary feasibility evaluation of m-DAC
For the initial investigation of the feasibility of m-DAC, we assumed a membrane with a permeance of 40,000 GPU and selectivity of 70, for which performances have been separately achieved in different membranes [18, 19]. Aspen Plus V11 (AspenTech, USA) flowsheeting software with the Peng–Robinson state equation thermodynamic model was used. The membrane separation process was modeled using the third-party unit operation MEMSIC, acquired from the University of Lorraine [20]. The following conditions were used for the process simulation:
-
(1)
The CO2 concentration of the retentate gas should be ~300 ppm, which is the level in the preindustrial period.
-
(2)
The pressures of the feed and permeate sides are 101.3 kPa (atmospheric pressure) and 5 kPa, respectively.
-
(3)
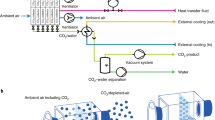
Multistage separation with four membrane separation units connected in series was considered (Fig. 1).
-
(4)
The process is driven by vacuum pumps, which are units of the process that require energy.
-
(5)
The outcomes of the energy requirements and required membrane area are normalized on the mass of CO2 in the product stream.
Simplified scheme of the multistage membrane separation model used for process simulation in Aspen Plus (process constraints: retentate CO2 = 300 ppm at every separation stage, feed pressure pf ~ 101 kPa (~1 atm), permeate pressure pp = 5 kPa, pressure ratio, φ ≈ 20; CO2/X selectivity of the membranes was considered equal to 70, where X identifies other components of air (N2, O2, Ar)
The results of the process simulation are summarized in Table 1. The relevant CO2 concentration of >10% in the permeate is reached by three- and four-stage separations, while single- or two-stage separation remains below 3% CO2. Concerning the membrane area, the required membrane area for four-stage separation is only ~3.2 m2/kg CO2/day as a flat sheet. This membrane area can be assembled into the membrane module with a volume of less than 0.01 m3 (for 1 kg-CO2 capture per day) estimated from the empirical membrane surface-to-volume ratio for plate-and-frame modules (~100–150 ft2/ft3) [21]. Notably, the amount of CO2 emissions estimated from the energy used by the separation process is only ~0.6 kg/1 kg-CO2 captured, leading to negative emission of CO2. These simple process simulation results encouraged us to explore more realistic membrane performances and process conditions for m-DAC.
Key parameters in the CO2 separation by membranes
In the membrane separation process, both membrane properties and process parameters play an important role in determining the overall performance of the process. In addition to the gas permeance and selectivity of membranes, stage cut and the pressure ratio between the feed and the permeate sides are important.
Gas permeance
The gas permeance property is important because the required membrane area is inversely proportional to this value. Therefore, only high permeance separation membranes can be considered as a practical option for DAC with realistic size and pressure conditions. It is noteworthy that membranes with high permeance would allow for the building of multistage separation processes with a reasonable membrane size, as shown in Table 1.
CO2 selectivity of membranes
The selectivity of membranes for CO2 capture is also critical. Single-step membrane separation can hardly exceed more than 1% of 400 ppm CO2 in air, even with a high CO2/N2 selectivity of 70. However, the high CO2 permeance of the membrane enables the application of multistage separation, making it feasible even with relatively low membrane selectivity for CO2 over other gas species. Figure 2a shows a three-stage membrane separation process used to analyze the effect of the CO2 selectivity on the CO2 purity in the product gas. The CO2 permeances of all membranes are assumed to be 10,000 GPU. The pressures of the feed and permeate sides at each separation step are 110 and 2 kPa, respectively. The CO2 selectivity of membranes over other gases is a variable parameter, but the same membrane performances at every stage are used for the analysis.
a Scheme of the three-stage membrane process for CO2 separation from air. b Dependence of CO2 purity in the product stream on membrane selectivity. c Requirements for the vacuum pump power (circles) and total daily energy/per kg of CO2 (diamonds) depending on membrane selectivity (the setting for pressure ratio: φ = 50)
In this model, the membrane size was adjusted to maintain the CO2 concentration of 300 ppm in the retentate of each stage. As seen in Fig. 2b, the final CO2 concentration does not exceed more than 10% when the CO2 selectivity is less than 20, even after three-stage separation. Higher CO2 selectivity results in higher CO2 concentrations in the permeate gas. However, the enhancement of CO2 purity in the product becomes moderate for the selectivity of more than 30. The required power of the pump at the first stage is the largest, and subsequent separation stages consume less energy since the gas volume after the first stage is significantly reduced (Fig. 2c). Higher CO2 selectivity contributes to the reduction of the pump energy, although the reduction rate becomes lower at selectivities greater than 30 (the black diamonds in Fig. 2c).
These results clearly show that the CO2 selectivity over other gases has a definitive and critical impact on the performance of m-DAC.
Pressure ratio
Process conditions also play an important role in determining the process performance in addition to the intrinsic membrane properties. The pressure ratio (φ), defined in Eq. (1), is a particularly important factor in the membrane separation process [22]
where \(p_f\) and \(p_p\) are the total pressure at the feed and permeate sides of the membrane. By considering the mass balances in a single membrane separation process, the partial pressure of CO2 in the permeate cannot exceed the partial pressure of CO2 in the feed; therefore, the following equation should be satisfied:
where \(C_p\) and \(C_f\) are the mole fractions of CO2 at the permeate and feed side, respectively. Hence, the following relation defines the permeate concentration with feed concentration and pressure ratio:
This equation implies that the pressure ratio limits the mole fraction of CO2 at the permeate side, independent of the membrane selectivity. Figure 3a shows the effect of the pressure ratio on the CO2 concentration at the permeate (product) and the retentate sides in the single-step separation from the air. The small pressure ratio has a large impact on the CO2 concentration in the permeate and retentate streams, but the trend plateaus in the high-pressure ratio region (φ > 30). This result demonstrates the importance of the pressure ratio in determining the CO2 concentrations after membrane separation. The pressure ratio is also related to the energy needed for vacuuming. Figure 3b shows the required energy (per kg-CO2), and there is an optimum pressure ratio for having local minimum energy at pressure ratios of 10–15, which is similar to the optimum pressure ratio for postcombustion CO2 capture [15].
Influence of the pressure ratio φ and stage cut (θ) on the CO2 separation from the air using a CO2 selective membrane. a Dependence of CO2 concentration in permeate and retentate streams and b CO2-mass normalized daily energy requirements on pressure ratio (other parameters: stage cut θ ~ 5–10 and selectivity CO2/X = 30, where X identifies other components of air (N2, O2, Ar). c Dependence of CO2 concentration in permeate and retentate streams and d CO2-mass normalized daily energy requirements on stage cut (other parameters: pressure ratio, φ = 20; selectivity CO2/X = 30, where X identifies other components of air (N2, O2, Ar)
Stage cut
In membrane separation, the feed gas flows along the membrane surface, and the CO2 concentration gradually decreases since CO2 selectively permeates across a membrane, leading to a reduced driving force for permeation. In contrast, the concentrations of other (less permeable) gases in the residue stream are relatively sustained, resulting in a stable driving force. Especially for high gas-permeable membranes, this effect becomes increasingly significant. A larger membrane area and slower feed flow allow for more efficient permeation of gas across a membrane. Therefore, the degree of separation has a trade-off relationship between purity and recovery that can be controlled by the flow rates and membrane area, which is known as the stage cut (\(\theta\)), which is defined as the ratio of permeate to feed flow. Figure 3c shows the effect of stage cut on the CO2 concentration of the permeate and feed sides. In the region of a relatively small stage cut, a higher CO2 concentration at the permeate side is obtained. However, the portion of CO2 separated from the feed gas (recovery rate) inevitably decreases and vice versa for relatively large stage cuts. Specifically, one can see that product purity and recovery of CO2 from air show a trade-off relationship (Fig. 3c, d). The stage cut factor can be controlled by the membrane area (at a stable feed flow) and should be optimized to achieve a target separation.
The design strategy of m-DAC and sensitivity analysis
By considering the importance of the factors described above, the membrane process for DAC was investigated as follows.
Target CO2 concentration for m-DAC
CO2 capture is one component of the process of CO2 capture, utilization, and sequestration (CCUS). Thus, the target conditions for CO2 capture depend on the subsequent CO2 treatment process. For transport and geological sequestration, a nearly pure CO2 stream is required [23]. On the other hand, there are no specific concentration requirements for CO2 utilization. A high concentration of CO2 is certainly desirable in CO2 conversion reactions to value-added materials. However, high purity at the CO2 separation step is not necessary if subsequent conversion steps can still proceed efficiently. Indeed, Kim et al. showed the potential for electrochemical conversion of low-purity CO2 [24]. They used CO2 gas diluted with N2 as a feedstock for the electrochemical reduction of CO2 and achieved efficient conversion reactions to CO, even at feed CO2 concentrations as low as 20%. Kumagai et al. also achieved CO2 conversion even at only 1000 ppm CO2 [25]. These results make it apparent that it is not necessary to produce a high-purity CO2 stream by membrane separation. Based on these considerations, for the subsequent analysis, the target CO2 purity in the final gas was set to 40%, which is 1000 times its concentration in the air.
Stage cut and pressure ratio
The main purpose of DAC is to reduce the CO2 concentration in the air by removing historical emissions and to offset the emissions that are difficult to avoid. Generally, membrane separation emits a retentate gas to the external air. Therefore, the CO2 concentration of the retentate gas should be less than 300 ppm (characteristic atmospheric CO2 concentration of the preindustrial period) by adjusting the stage cut (controlled by membrane area).
The pressure ratio also impacts the total performance of the DAC membranes. As shown in Fig. 3b, the pressure ratios ranging from ~10 to 20, which is a local minimum of the consumed energy, would be reasonable for analysis. However, the final concentration of CO2 within this pressure ratio range cannot reach 40% even after four-stage separation. When the pressure at the permeate side is 4 kPa (φ = 25), the final CO2 concentration can exceed 40% (Table 2). Although the final concentration of CO2 also depends on the membrane selectivity, as shown in Fig. 3a, pressure ratios higher than 30 in the membrane separation seem to be needed.
Influence of the gas permeance and selectivity on the m-DAC performance
Once the concentration of retentate CO2 concentration is defined, the volume of the permeate gas is simultaneously determined according to mass-balance constraints. The size of a membrane with relatively high gas permeance becomes small and vice versa if the separation parameters are identical. Therefore, the high gas permeance contributes to the reduction of the required membrane size and allows for one to build relatively small modules for multistage separation with practical sizes.
Membrane selectivity to CO2 also plays an important role in building a feasible m-DAC process. As shown in Fig. 2, membranes with CO2 selectivity of more than 70 can achieve the target CO2 concentration of 40% with a pressure ratio of 22 (in the three-stage process). However, the total performance of m-DAC depends on the parameters described above, and there may be a window of CO2 selectivity to satisfy the current target (1000 times CO2 concentration). To explore the range of CO2 selectivity, the following conditions were considered.
-
(1)
The pressures at the feed and permeate sides are 110 and 2 kPa, respectively (φ = 55).
-
(2)
The CO2 concentration of the retentate gas is set to 300 ppm.
-
(3)
Four-stage separation is considered (similar to Fig. 1).
-
(4)
The CO2 permeance is 10,000 GPU.
-
(5)
The CO2 selectivities over N2, O2, and Ar are assumed to be identical.
The obtained results are standardized for capturing 1 kg of CO2 per day.
As summarized in Table 3, membrane separation with a relatively realistic CO2 selectivity of 30 can reach the target CO2 concentration (more than 40%). The total amount of CO2 emissions through this process is less than 1 kg, leading to negative emission of CO2 with a membrane size of less than 5 m2/kg-CO2/day. In the separation with relatively low selectivity, other gases also permeate faster, resulting in a rapid increase in the CO2 concentration of the retentate stream. Thus, the area of the membrane with lower selectivity should be smaller than that with higher selectivity to release the retentate gas with a CO2 concentration of less than 300 ppm. Highly CO2 selective membranes show relatively lower energy for vacuuming and CO2 emission through the DAC process.
These analyses imply that there is space to achieve the current target, 1000-fold preconcentration of the CO2 from air, by optimizing parameters, which has never been considered before. As a short summary, the membrane performances and process factors need to satisfy the following conditions:
-
(1)
CO2 permeance: more than 10,000 GPU to achieve realistic membrane sizes.
-
(2)
CO2 selectivity >30 (higher selectivity is preferred).
-
(3)
A pressure ratio >30 (a higher pressure ratio is preferred).
One can claim that the discussion of CO2 capture in kg mass range may be negligible to reduce the CO2 concentration in the air since the amount of already emitted CO2 is incredibly large. However, once we have such membranes, they can be considered for relatively large CO2 capture facilities. For example, the required membrane for one ton of CO2 capture per day is estimated to be less than 5000 m2, leading to less than 15.2 m3 (~2.5 m × 2.5 m × 2.5 m in size) for the volume of the membrane unit. The massive installation of such membrane separation units would therefore have the potential to reduce the CO2 concentration in the air meaningfully.
In the present analysis, the electricity generated by the photovoltaic system was considered for m-DAC operation and to estimate the amount of CO2 emissions through the process. The price of PV electricity was $0.068/kWh in 2019 and predicted to average $0.039/kWh in 2021 [26], meaning that the cost of m-DAC can also be expected to fit within the socially acceptable price range. This paper aimed to evaluate the technical aspects of m-DAC and exploring the required membrane performances, and the analysis of the cost performance was not the main purpose. In addition, a large number of scenarios concerning electricity should be considered for detailed techno-economic analysis, including not only operational expenses but also capital expenses. Therefore, we did not examine the cost aspect of m-DAC in this paper. The reported estimates of cost (energy) of other DAC technologies vary from 50 to 1000 $/t-CO2, and the most detailed report describes the DAC process used by Carbon Engineering Ltd. (CE) relying on CO2 capture by strong bases (KOH) interacting with air through the plastic contactor. The levelized cost of CO2 capture from 94 to 232 $/t-CO2 was reported and was strongly dependent on financial assumptions and energy cost [13]. The CE design required 8.81 GJ of natural gas or 5.25 GJ of gas and 366 kWhr of electricity per t-CO2. Earlier studies of similar processes provided costlier estimates, namely, 610 and 780 $/t-CO2 in optimistic and realistic scenarios, respectively, [5]. Another recent report provided an estimate of the energies required in solid adsorbent-based DAC varying from ca. 15 to 60 MJ/kg-CO2 (4.5–17 kWhr/kg-CO2) [12], and these energy requirements are similar to those described here for m-DAC.
Concluding remarks
In general, the DAC process has been considered in form of large-scale systems to process a vast amount of air efficiently. Although there is no question about deploying large and integrated systems for reducing the CO2 concentration in the air, expensive initial costs and specific site conditions for the plant installation limit the rapid and massive deployment of DAC systems. To meet the recommendation from the IPCC for achieving the 1.5 °C scenario, other approaches should be proposed as much as possible. In this context, membrane separation has several advantages compared to the conventional sorbent-based DAC process. Generally, membrane separation is considered the most energy-efficient technique for CO2 separation among the various separation technologies. Moreover, this approach does not require special chemicals or sorbents for CO2 capture. The greatest advantage is that membrane separation systems are scalable and can be installed in a variety of locations. These characteristics of membrane separation can enable m-DAC to be the most suitable technology for ubiquitous and ambient DAC. This uniqueness of m-DAC provides opportunities to capture CO2 that have never been considered before. For example, the CO2 concentration in the school classroom often exceeds more than 1000 ppm without ventilation since human beings emit CO2 at a rate of ~1 kg per person per day [27]. In such places of higher CO2 concentrations, CO2 capture by membranes becomes relatively efficient since the driving force of CO2 is larger. Recently, Dittmeyer et al. discussed the potential of an air conditioner system with a CO2 capture function [28]. Their argument is based on existing DAC technologies that use CO2 absorption and adsorption. The energy cost of CO2 recovery is still high and it is essentially identical to the large-scale system, and specific chemicals for CO2 capture are required. This situation makes it difficult to introduce conventional systems into offices and private residences. Therefore, for a truly distributed and scalable DAC system, m-DAC would be the most suitable technology.
As discussed above, the permeance of CO2 and selectivity toward other gases (N2, O2) together with the process parameters (pressure ratio and stage cut) will define the degree of preconcentration and required energy in the m-DAC system. Membrane materials play a large role in capital investments, as the required membrane areas are relatively large. Therefore, the materials used should be suitable for thin-film fabrication. Considering the specific materials, thermally reversed polymers and polymers of intrinsic microporosity (PIMs) have shown superior CO2 permeability, breaking the upper bound [29], but higher CO2 selectivity is desired. Moreover, PIMs manifest accelerated physical aging when fabricated as submicron-thick membranes [30]. By combining specific fillers and polymers, mixed-matrix membranes (MMMs) can reach both high CO2 permeability and selectivity. However, for the m-DAC process to be practical, the permeance is the key parameter (not permeability), which leads to the requirement of ~100 nm membrane thicknesses. Most MMMs cannot be fabricated at this thickness. Facilitated transport membranes are promising, especially for the first step of separation with a low concentration of CO2 in the feed, but may lack performance in later stages where the CO2 concentration is higher.
Although the target properties of membranes for m-DAC are challenging, recently, a thin-film composite membrane approach was used to improve the performance [30, 31]. In this approach, the CO2 selective thin layer is deposited on the highly gas-permeable support layer, the so-called gutter layer, and assembled on a porous support (Fig. 4). We believe it is promising to provide membranes for m-DAC because the layers are often made of versatile, low-cost polymers that can be fabricated with thicknesses of a few tens of nanometers without losing the performance [18], while the selectivity can be improved by surface modification [19]. Moreover, for m-DAC, it is important to study all materials that show promising bulk performance in the form of thin membranes or thin composites to determine whether the separation is influenced by the thickness and whether large-scale, mechanically stable membranes can be fabricated.
In addition, CO2/O2 separation becomes important, especially for the CO2 conversion process, since oxygen prevents efficient CO2 reduction [32]. To the best of our knowledge, CO2/O2 separation by membranes has rarely been addressed in the literature, and most previous research on membrane-based CO2 separation was devoted to postcombustion capture (separation of CO2 and N2), precombustion capture (CO2 and H2), and natural gas sweetening (CO2 and CH4). Although much research is related to oxygen-enriching membranes, for example, that based on oxygen ion conductive materials, silicone-based polymers, or other organic polymers, most of the studies focus on O2 separation over N2 and do not report preferential CO2 permeation over O2. All CO2 capture technologies, including this m-DAC process, should be combined with successive processes to manage captured CO2. Chemical conversion is one of the major processes to circulate CO2 as a carbon source. In the process of CO2 conversion, it is chemically reduced to other value-added compounds, such as CO and CH4. O2 inhibits the conversion of CO2 to the corresponding reduced form since it is a strong oxidant. Therefore, the product gas from the m-DAC system should not only achieve a relevant concentration of CO2 but also significantly reduce concentrations of O2, which emphasize the requirement of high CO2/O2 selectivity, in addition to CO2/N2 selectivity, although such a separation situation has never been considered.
As described above, the CO2 capture process is one component of CCUS and thus the subsequent process after DAC by membranes should be considered carefully. The target performance of the membrane for DAC depends on the requirements in the subsequent utilization processes. The simplicity of membrane separation offers many opportunities to capture and utilize CO2, and this versatile ability of the m-DAC process has great potential to establish a future sustainable and circular economy enabled by carbon recycling and negative carbon emissions.
Change history
18 November 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Masson-Delmotte V, Zhai P, Pörtner H-O, Roberts D, Skea J, Shukla PR, et al. Summary for Policymakers. In: Global warming of 1.5 °C. An IPCC special report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. IPCC; 2018. https://report.ipcc.ch/sr15/pdf/sr15_spm_final.pdf.
Kriegler E, Luderer G, Bauer N, Baumstark L, Fujimori S, Popp A, et al. Pathways limiting warming to 1.5 °C: a tale of turning around in no time? Philos Trans R Soc A Math Phys Eng Sci. 2018;376:20160457.
Fuss S, Canadell JG, Peters GP, Tavoni M, Andrew RM, Ciais P, et al. Betting on negative emissions. Nat Clim Change. 2014;4:850–3.
Gasser T, Guivarch C, Tachiiri K, Jones CD, Ciais P. Negative emissions physically needed to keep global warming below 2 °C. Nat Commun. 2015;6:7958.
Socolow R, Desmond M, Aines R, Blackstock J, Bolland O, Kaarsberg T, et al. Direct air capture of CO2 with chemicals panel on public affairs. Am Phys Soc. 2011;100:1–91.
Sanz-Pérez ES, Murdock CR, Didas SA, Jones CW. Direct capture of CO2 from ambient air. Chem Rev. 2016;116:11840–76.
Keith DW. Why capture CO2 from the atmosphere? Science. 2009;325:1654–5.
National Academies of Sciences, Engineering, and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda. Washington, DC: The National Academies Press; 2019. https://doi.org/10.17226/25259.
Smith P, Davis SJ, Creutzig F, Fuss S, Minx J, Gabrielle B, et al. Biophysical and economic limits to negative CO2 emissions. Nat Clim Change. 2016;6:42–50.
Minx JC, Lamb WF, Callaghan MW, Fuss S, Hilaire J, Creutzig F, et al. Negative emissions—part 1: research landscape and synthesis. Environ Res Lett. 2018;13:063001.
Fuss S, Lamb WF, Callaghan MW, Hilaire J, Creutzig F, Amann T, et al. Negative emissions—part 2: costs, potentials and side effects. Environ Res Lett. 2018;13:063002.
Stampi-Bombelli V, van der Spek M, Mazzotti M. Analysis of direct capture of CO2 from ambient air via steam-assisted temperature–vacuum swing adsorption. Adsorption. 2020. https://doi.org/10.1007/s10450-020-00249-w.
Keith DW, Holmes G, St. Angelo D, Heidel K. A process for capturing CO2 from the atmosphere. Joule. 2018;2:1573–94.
Baker RW, Freeman B, Kniep J, Huang YI, Merkel TC. CO2 capture from cement plants and steel mills using membranes. Ind Eng Chem Res. 2018;57:15963–70.
Merkel TC, Lin H, Wei X, Baker RW. Power plant post-combustion carbon dioxide capture: an opportunity for membranes. J Memb Sci. 2010;359:126–39.
Keith DW, Heidel K, Cherry R. In: Brian L, editor. Geo-engineering climate change. Environment necessity or Pandora’s box? Capturing CO2 from atmosphere: rationale and process design considerations. (Chapter 6). Cambridge University Press; 2010. p. 107–26.
Yoo MJ, Kim KH, Lee JH, Kim TW, Chung CW, Cho YH, et al. Ultrathin gutter layer for high-performance thin-film composite membranes for CO2 separation. J Memb Sci. 2018;566:336–45.
Fujikawa S, Ariyoshi M, Selyanchyn R, Kunitake T. Ultra-fast, selective CO2 permeation by free-standing siloxane nanomembranes. Chem Lett. 2019;48:1351–4.
Selyanchyn O, Selyanchyn R, Fujikawa S. Critical role of the molecular interface in double-layered Pebax-1657/PDMS nanomembranes for highly efficient CO2/N2 gas separation. ACS Appl Mater Interfaces. 2020;12:33196–209.
Bounaceur R, Berger E, Pfister M, Ramirez Santos AA, Favre E. Rigorous variable permeability modelling and process simulation for the design of polymeric membrane gas separation units: MEMSIC simulation tool. J Memb Sci. 2017;523:77–91.
Li D, Wang R, Chung T-S. Fabrication of lab-scale hollow fiber membrane modules with high packing density. Sep Purif Technol. 2004;40:15–30.
Huang Y, Merkel TC, Baker RW. Pressure ratio and its impact on membrane gas separation processes. J Memb Sci. 2014;463:33–40.
IPCC. IPCC special report on carbon dioxide capture and storage. Metz B, Davidson O, de Coninck HC, Loos M, Meyer LA, editors. Prepared by Working Group III of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. p. 442.
Kim B, Ma S, Molly Jhong H-R, Kenis PJA. Influence of dilute feed and pH on electrochemical reduction of CO2 to CO on Ag in a continuous flow electrolyzer. Electrochim Acta. 2015;166:271–6.
Kumagai H, Nishikawa T, Koizumi H, Yatsu T, Sahara G, Yamazaki Y, et al. Electrocatalytic reduction of low concentration CO2. Chem Sci. 2019;10:1597–606.
IRENA. Renewable power generation costs in 2019. IRENA; 2020. https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Jun/IRENA_Power_Generation_Costs_2019.pdf.
Ranjan M, Herzog HJ. Feasibility of air capture. Energy Procedia. 2011;4:2869–76.
Dittmeyer R, Klumpp M, Kant P, Ozin GA. Crowd oil not crude oil. Nat Commun. 2019;10:1818.
Comesaña-Gándara B, Chen J, Bezzu CG, Carta M, Rose I, Ferrari MC, et al. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ Sci. 2019;12:2733–40.
Selyanchyn R, Fujikawa S. Membrane thinning for efficient CO2 capture. Sci Technol Adv Mater. 2017;18:816–27.
Xie K, Fu Q, Qiao GG, Webley PA. Recent progress on fabrication methods of polymeric thin film gas separation membranes for CO2 capture. J Memb Sci. 2019;572:38–60.
Williams K, Corbin N, Zeng J, Lazouski N, Yang DT, Manthiram K. Protecting effect of mass transport during electrochemical reduction of oxygenated carbon dioxide feedstocks. Sustain Energy Fuels. 2019;3:1225–32.
Federation of Electric Power Companies of Japan. Energy and Environment 2016. Federation of Electric Power Companies of Japan; 2017. https://www.fepc.or.jp/english/library/energy_environment/__icsFiles/afieldfile/2017/12/11/env_ene2016e.pdf.
Acknowledgements
This work was supported by the World Premier International Research Center Initiative (WPI), sponsored by the Japanese Ministry of Education, Culture, Sports, Science, and Technology. SF acknowledges the Japan Society for Promotion of Science (JSPS) for a Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI Grant Number JP20H02781). RS acknowledges the Japan Society for Promotion of Science (JSPS) for a Grant-in-Aid for Early Career Scientists (JSPS KAKENHI Grant Number JP19K15342).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Full information regarding the change(s) made can be found in the correction for this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujikawa, S., Selyanchyn, R. & Kunitake, T. A new strategy for membrane-based direct air capture. Polym J 53, 111–119 (2021). https://doi.org/10.1038/s41428-020-00429-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00429-z
This article is cited by
-
Synthesis, characterization, and photoreduction performance evaluation of gold/titanium oxide/calcium carbonate photocatalysts for carbon dioxide reduction
SN Applied Sciences (2022)
-
Special issue: CO2: capture of, utilization of, and degradation into
Polymer Journal (2021)
-
Solar methanol energy storage
Nature Catalysis (2021)