Abstract
When the radical polymerization inhibitor 1,4-naphthoquinone (NQ) acts on styrene, the radical capture reaction greatly contributes carbon atoms. In this study, the 1-phenethyl radical, which is a model for the growth radicals of polystyrene, was reacted with NQ using four radical sources, and we identified the reaction product. We confirmed that the same compound was formed upon addition of a small amount of NQ to heated styrene. The frontier orbital energy levels and reaction path energy diagrams were calculated using density functional theory with Gaussian 09, which provided numerical values to support our experimental results.
Introduction
Polymerization inhibitors are substances that are added to a monomer to prevent spontaneous polymerization. Usually, when a polymerization reaction progresses, thickening and polymerization heat generation occur [1, 2]. If the reaction is not sufficiently controlled, there are risks of deterioration and accidents. Therefore, a polymerization inhibitor for stabilization is added for storing the radical polymerizable monomer. Among potential inhibitors, we direct our research toward an unsaturated polyester resin based on a styrene monomer and focus on 1,4-naphthoquinone (NQ), which is used worldwide as a polymerization inhibitor. NQ has unique inhibitor advantages, such as an ability to secure long-term storage stability, a lack of curing retardation, and an improved gel time drift [3, 4]. Thus, NQ exhibits prohibitive behavior that is clearly different from those of phenolic and stable radical polymerization inhibitors.
The characteristics of the styrene monomer include radical generation reactions by heat or light. Mayo and Flory showed that after the [4 + 2] cyclization and biradicalization of two molecules of styrene occur, a hydrogen atom transfer reaction with another monomer generates a phenethyl radical (1) and dimer radicals (2, 3) [5, 6]. By the generation of these radical species shown in Scheme 1, the polymerization naturally initiates, even without an initiator [7, 8].
Representative radical polymerization inhibitors include phenols such as TBC (tertiary-butyl catechol) and stable nitroxyl radicals such as TEMPO (2,2,6,6-tetramethyl piperidine-N-oxyl). The inhibiting effect of the phenolic polymerization inhibitors arises from the hydrogen atom transfer reaction of the OH group [9], and the inhibiting effect of the stable radical polymerization inhibitor arises from quenching of the active species by a radical addition reaction [10].
The inhibitors 1,4-benzoquinone (BQ) and NQ (Fig. 1), which are part of the quinone compound group, exhibit good polymerization inhibiting effects, such as the aforementioned compounds. In general, the inhibiting mechanism of BQ is an addition reaction of growth radicals on the BQ carbonyl oxygen atom [11].
The polymerization inhibition reaction of BQ to styrene was exemplified by Engel et al. [12]. These researchers conducted a model experiment to react BQ with 1 using the radical precursor azobis(1-phenylethane) (4) and found that the main reaction is the addition reaction on the carbonyl oxygen. The main product of this reaction is the diether or monoether form of hydroquinone. In addition, Sharma et al. [13] found that methyl radicals are substituted on NQ with methyl groups on the carbons in the 2- and 3-positions. This reaction mechanism adds a methyl radical to the NQ ring, and then, a hydrogen atom transfer reaction with the methyl radical of another molecule occurs.
From the viewpoint of organic synthetic chemistry, substituted BQs and NQs, such as vitamin K, are useful compounds, and the synthesis of quinone derivatives is well studied [14, 15]. It is widely known that substituted NQs can be synthesized by reaction with an alkyl radical, which has been demonstrated in various recent radical generation methods with NQ reacting at the carbons in the 2- and 3-positions [16,17,18]. However, there are no examples of studies on the reactivity of 1 on NQ. The mechanism of radical polymerization inhibition of NQ against styrene is unknown, and elucidation of its mechanism of action is still necessary.
In this study, we attempted to elucidate the polymerization inhibition mechanism of NQ by using three methods: product identification by model reaction of NQ with the phenethyl radical 1 under various conditions, gel permeation chromatography (GPC) and liquid chromatography–mass spectrometry (LC/MS) analyses of a stored sample of styrene monomer with NQ, and computational chemistry analysis. These methods allowed us to reveal the polymerization inhibition mechanism of NQ.
Experimental procedure
Measurements
The UV–vis spectra were recorded on a JASCO V-550 UV/VIS spectrophotometer (MeCN solvent, 1 -cm path length). The FT-IR spectra were recorded on a Nicolet iS10 FT-IR Spectrometer (KBr pellet method). The 1H and 13C-NMR spectra were recorded on BRUKER DRX-300 and JEOL ECX-400 instruments. The LC/MS spectra were recorded on a Shimadzu LC-2010C HT and Shimadzu LCMS-2020, column: Wakopack Navi C18-5 φ 2.0 × 150 mm (D), with an MeCN/H2O gradient as the solvent. The GPC analysis was performed on a JASCO RI-2031 Plus, column: TOSOH TSK-gel G200 HXL φ 7.8 × 300 mm, with four columns connected in series and THF as a solvent.
Reaction of NQ with α-phenethyl radical (1)
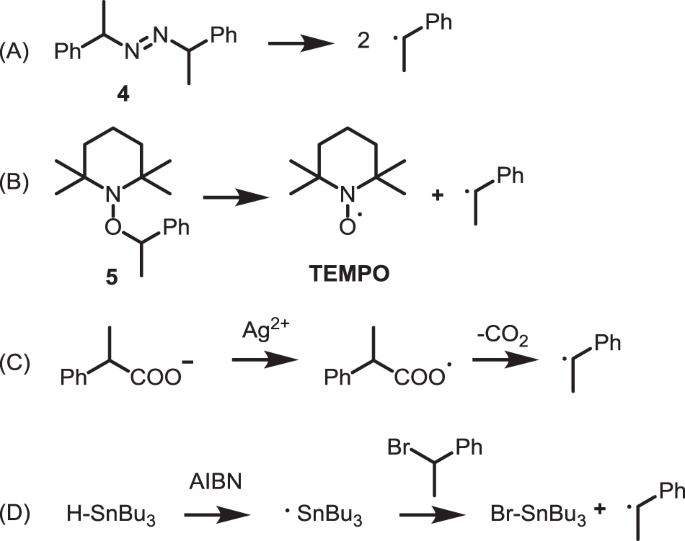
A model experiment was conducted in which α-phenethyl radical (1) generated in the self-reaction of styrene was regarded as a radical for growth at the polymerization end and reacted with NQ. The reaction position of NQ was thereby determined. In this study, the four methods shown in panels a–d of Fig. 2 were used as methods to generate 1. After the reaction, appropriate treatments and isolation of silica gel by TLC and flush column chromatography were carried out.
Azobis(1-phenylethane) (4), which was used as the first radical source, was synthesized by methods from the literature [19, 20]. Cohen et al. reported that 4 decomposes when heated above 100 °C and generates 1 [21]. NQ and 4 were dissolved in tert-butyl benzene and reacted at 120 °C for 14 h (Method A).
A TEMPO derivative (2,2,6,6-tetramethyl-N-(1-phenylethoxy) piperidine, 5 was used as the second radical source and was synthesized using a method from the literature [22]. In this compound, the C–O bond dissociates when heated and decomposes into TEMPO and 1. NQ and 5 were dissolved in tert-butyl benzene and reacted at 120 °C for 14 h (Method B).
As the third radical source, we used the radical generation reaction via oxidation of carboxylic acid ions by Ag(I) and S2O82− reported by Underson et al. [23] This source is a reaction of NQ with 1 under the presence of an oxidizing agent. NQ and α-phenylpropionic acid were added to a mixed solvent (H2O/MeCN = 2/3), and the reaction was carried out at 60 °C for 3.5 h with gradually decreasing S2O82− (Method C).
As the fourth radical source, we used a radical generation method [24] with HSnBu3 and alkyl bromide. This source is a reaction of NQ with 1 under the presence of a hydrogen donor (reducing agent). AIBN and 1-bromo-1-phenylethane were added to benzene, and the reaction was carried out at 80 °C for 4.5 h (Method D).
Polymerization of styrene monomer in the presence of NQ
An experiment was conducted to ascertain whether the same reaction occurred in the styrene monomer. Commercially available styrene monomer was refined by alkaline washing. An accelerated test was performed by adding 1 wt% or 5 wt% NQ and storing the sample sealed and under light shielding at 80 °C. Samples were collected every other week until 4 weeks had passed. Then, samples were diluted with the proper solvent and analyzed by GPC and LC/MS. The presence or absence of each compound was confirmed by comparison with the retention time of the purified product in the previous section.
Computational study
To enable a theoretical discussion of the experimental results, a computational study was conducted using the molecular orbital calculation program Gaussian 09 [25]. The initial structure used for the input files was generated by a conformational search of a MM3 molecular force field calculation that ran 2000 times using the Medit [26] and Winmostar [27] programs. The initial transition-state structures were generated by the minimum energy path method at the PM3 level. Using these initial structures as input files, we optimized the structures with the basis function of (U)B3LYP/6-31G* in Gaussian 09, and various quantities were calculated by single-point calculations on the (U)B3LYP/6-311+G** level.
Results and discussion
Reaction of NQ with α-phenethyl radical (1)
For Method A, an isolation operation was carried out using PTLC (preparative thin layer chromatography) on the crude product of the reaction of NQ with 4. The main isolated product was identified as 2-(1-phenylethyl)-1,4-naphthoquinone (6) from 1H-NMR, 13C-NMR, FT-IR, and ESI-MS (m/z = 262) (see the Supplementary information). As a byproduct, 1,4-bis(1-phenylethoxy)naphthalene (7), which is a radical adduct on the carbonyl oxygen, was obtained (Fig. 3). For Method B, 1 was released by decomposition of TEMPO derivative 5 and reacted with NQ, resulting in generation of 6. The product 6 was more stable than TEMPO derivative 5. Even under the coexistence of oxidizing and reducing agents, the main product of NQ was 6. For Method C, 6 was obtained with a yield of 29%. In Method D, 6 was obtained with a yield of 38%.
From Methods A–D, we showed that 1 mainly reacts with the 2-position carbon of NQ and forms 6 (Table 1). Although there were small amounts of other compounds that could not be isolated and structurally determined, 6 was the main product when the starting material NQ was recovered. For example, in the reaction between NQ and 1, the production of a monoether compound (4-(1-phenylethoxy)-1-naphthol, 8) is expected, but this species was not isolated in this experiment. In addition, in the LC/MS analysis of the crude product of Method A, a peak of m/z = 264 corresponding to 8 was not found.
Although the main product did not change, dependence of the product and the yield on the radical generator, such as 7, was observed only for Method A. This result was considered to be mainly due to the reaction temperature, the number of radicals present in the reaction, and the presence or absence of an oxidizing or reducing agent. As for the formation of 7, Methods A and B had higher temperatures than C and D. Compared with Method A, Method B was expected to recombine the radicals generated with TEMPO and reduce the number of radicals in the reaction. In Method A, the reaction temperature was higher, and a higher amount of radical 1 was present. As a result, 7 was generated, which would have been difficult in the other methods.
Polymerization of styrene monomer in the presence of NQ
The stored samples containing NQ added to the purified styrene were diluted with chloroform and analyzed by GPC, and the results are presented in Fig. 4a–d. In the sample to which 1 wt% NQ was added, the amount of NQ (retention time (r.t.) = 35 min) decreased from the first week (a) to the fourth week (b), and both peaks of polystyrene (r.t. = 20–25 min and r.t. = 32 min) increased. The peak at 32 min was presumed to be compound 6 since the retention time of 6 at this measurement condition was identified as 32 min by using synthesized 6. In the case of 5 wt% addition of NQ, polystyrene did not form from the first week (c) to the fourth week (d), and several peaks appeared in the r.t. range of 28–32 min. These peaks were considered to be a group of compounds in which NQ and oligomers of styrene were bonded, including compounds such as 6, 9, and 10 (Fig. 5).
The vertical axis in Fig. 4 is the absorption intensity of UV at 254 nm. Since both NQ and styrene have absorptions at 254 nm, we could not determine whether the compound of the detected peak was derived from NQ or styrene. NQ absorbs the wavelength of 330 nm, but styrene does not. When 330 nm was used as the detection wavelength, the NQ derivative could be identified. From the 330-nm analysis, a UV absorption peak at 330 nm was found at r.t = 20 min, r.t = 26–30 min, and r.t. = 32 min. The peak at 20 min was thought to be a compound in which NQ is bonded to the growth end of the polystyrene chain. The peak at 26–30 min was a compound in which the growth end of a styrene oligomer or radicals 2 and 3 are bonded to NQ, and the peak at 32 min was estimated to be compound 6.
In addition, samples (b) and (d) were analyzed by LC/MS. When (b) was diluted with MeCN as the solvent for LC, a white precipitate of polystyrene was formed. The precipitate was removed, and the supernatant was analyzed by LC/MS. Compounds at m/z = 262 and m/z = 364 were confirmed to exist in the region with lower polarity than NQ. On the other hand, no precipitate was formed when diluting sample (d). LC/MS analysis showed the presence of m/z = 262 and m/z = 364. Between these peaks, m/z = 262 was consistent with compound 6 in retention time. We presumed that m/z = 364 was a compound with a longer retention time and a lower polarity than 6. From the molecular weight, this compound was presumed to be 9 and/or 10, which is the product of the reaction between a styrene dimer radical (2, 3) and NQ. A peak at m/z = 368 corresponding to 7 was not observed.
Computational study
In this study, we revealed that when NQ reacts with 1, the main reaction point is on the 2-position carbon. We examined the reason why this result occurs. The HOMO and LUMO energy levels of the NQ- and styrene-derived radicals (1, 2, and 3), which were obtained by single-point calculations at the B3LYP/6-311+G** level after structure optimization at the B3LYP/6-31G* level, are shown in Fig. 6. The calculations showed that the frontier orbital levels of the three radicals have similar energy levels. This result means that NQ, having electrophilic properties, tends to react with radicals 1, 2, and 3, which have nucleophilic properties. According to Fukui et al. [28, 29], the radical reaction is more likely to occur on the atom where the coefficient fA(R) value is more localized. The fA(R) value can be estimated by adding the coefficients of the HOMO and LUMO to Eq. 1 in Fig. 6b. As shown in Fig. 6b, the fA(R) value of NQ is high on the 2-position carbon. The computational study supported the experimental finding that the main product obtained in this reaction is 6.
In addition to the predictions by frontier orbital theory, we composed an energy diagram with reaction intermediates and transition states, which is shown in Fig. 7. The transition-state structure had one imaginary vibration that was confirmed to be the desired vibrational mode.
The activation enthalpies of both TSO-1 and TSC-1 (Fig. 7) are small and are sufficient for the reaction to proceed even at room temperature. IntO and IntC are in an equilibrium state, and both exist at the initial stage of the reaction. The activation enthalpy from IntO to TSO-2 is 32.2 kcal/mol, whereas that from IntC to TSC-2 is 16.0 kcal/mol. The latter is approximately half of that of the former. Therefore, in the reaction between NQ and 1, the reaction proceeds via TSC-1 and TSC-2 and generates 6 as the main product, rather than via TSO-2. The cause of the difference in reactivity with BQ will be calculated in detail in the future.
Conclusion
We established that the phenethyl radical 1 reacts on the 2-positioned carbon of NQ by a model experiment using four kinds of radical sources and by a storage experiment of NQ and styrene monomer. This research has not yet elucidated all the reaction products of radicals and NQ generated in styrene. However, in both the model experiments using four kinds of radical sources and LC/MS analysis of the stored styrene samples with NQ, 6 was the main product of the reaction between NQ and 1. This result shows that the radical polymerization inhibiting ability of NQ is largely due to the quenching reaction of the radical species at the 2-position carbon of NQ. The frontier orbitals calculated by computational chemistry and the energy diagram of the reaction paths support the experimental results. More detailed research will be conducted on the differences in reactivity with other quinones in the future. We believe that these results will lead to the elucidation of the unique inhibitor advantages of NQ, including its ability to secure long-term storage stability, lack of curing retardation, and improved gel time drift. Based on our research findings, we anticipate new polymerization control approaches using NQ and the molecular design of improved polymerization inhibitors.
References
Cox WP, Merz EH. Correlation of dynamic and steady flow viscosities. J. Polym. Sci. 1958;28:619–22.
Fox TG, Flory PJ. The glass temperature and related properties of polystyrene. Influence of molecular weight. J. Polym, Sci. 1954;14:315–9.
Fink JK. Reactive polymers: fundamentals and applications. In: William A, editor. Unsaturated Polyester Resins. Oxford: William Andrew, Inc. 2005. p. 17.
Parker PH, Jr. U. S. patent 3300544A, 1965.
Mayo FR. The dimerization of styrene. J. Am. Chem. Soc. 1968;90:1289–95.
Flory PJ. The Mechanism of Vinyl Polymerizations. J. Am. Chem. Soc. 1937; 59:241–53.
Hui AW, Hamielec AE. Thermal polymerization of styrene at high conversions and temperatures. An experimental study. J. Appl. Polym. Sci. 1972;16:749–69.
Russell KE, Tobolsky AV. Thermal Initiation of Styrene Polymerization. J. Am. Chem. Soc. 1953;75:5052–4.
Liao CC, Wu SH, Su TS, Shyu ML, Shu CM. Thermokinetics evaluation and simulations for the polymerization of styrene in the presence of various inhibitor concentrations. J. Therm. Anal. Calorim. 2006;85:65–71.
Winter RAE, Li SS, Gatechair LR, von Ahn VH. US Patent 5254760A, 1993.
Cohen SG. Inhibition and Retardation of the Peroxide Initiated Polymerization of Styrene. J. Am. Chem. Soc. 1947;69:1057–64.
Engel PS, Park HJ, Mo H, Duan S. The reaction of α-phenethyl radicals with 1,4-benzoquinone and 2,6-di-tert-butyl-1,4-benzoquinone. Tetrahedron 2010;66:8805–14.
Sharma SC, Torssell K. Alkoxycarbonylation of quinones. A route to naphthacene quinones. Reversibility in homolytic substitution. Acta Chem. Scand. B 1978; 32:347–53.
Coppa F, Fontana F, Minisci F, Barbosa NMC, Vismara E. Homolytic alkylation of naphthoquinone and methyl-naphtoquinone. Enthalpic steric and polar effects. Tetrahedron 1991;47:7343–52.
Coppa F, Fontana F, Lazzarini E, Minisci F. A. New Selective Method for the Homolytic Alkylation and Carboxylation of Quinones by Monoesters of Oxalic Acid. Chem. Lett 1992;21:1299–302.
El-Hout SI, Suzuki H, El-Sheikh SM, Hassan HMA, Harraz FA, Ibrahim IA, El-Sharkawy EA, Tsujimura S, Holzinger M, Nishina Y. Tuning the redox potential of vitamin K3 derivatives by oxidative functionalization using a Ag(I)/GO catalyst. Chem. Comm. 2017;53:8890–3.
Hamsath A, Galloway JD, Baxter RD. Quinone C–H Alkylations via Oxidative Radical Processes. Synthesis 2018;50:2915–23.
Sutherland DR, Veguillas M, Oates CL, Lee AL. Metal-, Photocatalyst-, and Light-Free, Late-Stage C–H Alkylation of Heteroarenes and 1,4-Quinones Using Carboxylic Acids. Org. Lett. 2018;20:6863–7.
Daub GH, Cannizzo LF. A convenient synthesis of meso-azobis-.alpha.-phenylethane. J. Org. Chem. 1982;47:5034–5.
Bruch M, Jun YM, Leudtke AE, Schneider M, Timberlake JW. Synthesis of tetraisopropylethane and tetracyclopropylethane, and generation of the pentacyclopropylethyl carbocation. J. Org. Chem. 1986;51:2969–73.
Cohen SG, Groszos SJ, Sparrow DB. 1-Azo-bis-1-arylalkanes and their Decomposition. J. Am. Chem. Soc. 1950; 72:3947–51.
Zhou Z, Chen X, Holdcroft S. Stabilizing Bicontinuous Nanophase Segregation in πCP−C60 Donor−Acceptor Blends. J. Am. Chem. Soc. 2008;130:11711–8.
Anderson JM, Kochi KK. Silver(I)-catalyzed oxidative decarboxylation of acids by peroxydisulfate. Role of silver(II). J. Am. Chem. Soc. 1970;92:1651–9.
Giese B, Gonzalez-Gomes JA, Witzel T. The Scope of Radical CC‐Coupling by the “Tin Method”. Angew. Chem. Int. Ed. Engl. 1984;23:69–70.
Gaussian 09, Revision A.02, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al. Wallingford, CT: Gaussian, Inc.; 2009. http://gaussian.com/
Allinger NL. Molecular structure. Hoboken, NJ: Wiley; 2010.
James JPS, Frank J. Seiler Research Laboratory, U.S. Air Force Academy, Colorado Springs, CO 80840.
Fukui K, Yonezawa T, Shingu H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952;20:722–5.
Fukui K, Yonezawa T, Nagata C, Shingu H. Molecular Orbital Theory of Orientation in Aromatic, Heteroaromatic, and Other Conjugated Molecules. J. Chem. Phys. 1954;22:1433–42.
Acknowledgements
We are grateful to Associate Professor Azusa Kikuchi and Professor Kazuhisa Sakakibara (Department of Advanced Materials Chemistry, Yokohama National University) for the experimental advice. We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Takahashi, T., Ikejiri, Y., Himori, S. et al. Polymerization inhibition mechanism of 1,4-naphthoquinone by experimentation and DFT calculations. Polym J 51, 929–934 (2019). https://doi.org/10.1038/s41428-019-0206-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-019-0206-3