Abstract
Grass and wood biomass (bagasse, cedar, and eucalyptus) was directly converted into flame-retardant thermoplastics by dissolution in an ionic liquid mixture and successive precipitation. During dissolution, the hydroxy groups of the biomass were substituted with a phosphonate-type ionic liquid. The three resulting biomass samples were formed into thin films by hot pressing at 140–160 °C. They also self-extinguished a fire by forming foamed char layers after contacting the fire in an alcohol lamp. In this method, more than 86% of the hydroxy groups were maintained after ionic liquid substitution because the single phosphonate-type ionic liquid acted as a plasticizer and flame retardant in the plant biomass. Therefore, plant biomass-derived flame-retardant thermoplastics have the potential for further functionalization.
Similar content being viewed by others
Introduction
A large amount of plant biomass is produced as waste (e.g., bagasse and sawdust) in the industrial processes of food and manufacturing products, and the biomass has attracted attention as a renewable resource. However, the utilization of these biomass resources through chemical modification is quite difficult because of poor solubility of plant biomass. In most cases, plant biomass has been used after separation and refinement into each purecomponent: cellulose, hemicellulose, and lignin. Cellulose utilization has especially progressed over the past decades—for example, cellulose has been converted into thermoplastics by acylation [1,2,3,4]. Flame-retardancy is also important in the daily use of cellulose thermoplastics because these materials are flammable. Aoki et al. reported a flame-retardant cellulose thermoplastic synthesized by the adduction of a phosphoric acid-type flame retardant to the residual hydroxy groups of cellulose propionate (CP) [5].
On the other hand, we have reported flame-retardant cellulose thermoplastics just by dissolution in a single ionic liquid (salts that are liquids below 100 °C) species and precipitation (hemicellulose and lignin have also been thermoplasticized with flame retardancy through the same method) [6]. Specifically, the anion of 1-ethyl-3-methylimidazolium methylphosphonate ([C2mim][(MeO)(H)PO2]) [7, 8] is covalently introduced into the hydroxy group of cellulose, hemicellulose, or lignin simply by mixing and heating at a high temperature (Fig. 1). As a result, this method adds thermoplasticity and flame-retardancy, which are intrinsic effects of typical ionic liquids [9,10,11], to the samples and prevents bleeding out of the ionic liquid by covalent bonding. Furthermore, we have found that an ionic liquid combining a dialkylimidazolium cation and phosphonate anions acts as an intumescent flame retardant [6]. The intumescent flame retardant releases inert gases, such as N2, upon forming char from phosphorus compounds, resulting in foamed char layers. These foamed char layers are highly adiabatic, and thus, the cellulose, hemicellulose, and lignin substituted with [C2mim][(MeO)(H)PO2] (IL-cellulose, IL-hemicellulose, and IL-lignin) are able to self-extinguish [6]. There is an additional significant advantage to this method as follows. In the method reported by Aoki et al. (who introduced a phosphate-type flame retardant to CP) [5], the resulting samples cannot be further functionalized because almost all hydroxy groups are substituted by the flame-retardant and plasticizer groups, meaning that there are no more reactive groups in the polymers. Therefore, the further control of other properties of the cellulosic plastics which are important for molding and practical use (e.g., physico-chemical properties, such as viscosity and solubility, and mechanical properties, such as bending strength, tensile strength, and elastic modulus) cannot be achieved. On the other hand, more than 66% of the unreacted hydroxy groups are maintained in the method mentioned above, suggesting the potential for further functionalization to control the abovementioned properties [7].
From these results, flame-retardant thermoplastics from each component of biomass—cellulose, hemicellulose, and lignin—are ready to use. However, complicated purification is required to obtain each component from the plant biomass. In addition, all components cannot be recovered with high purities and yields. For example, an alkaline treatment followed by bleaching to extract pure cellulose causes the denaturation and decomposition of lignin [12] and may cause the decomposition of carbohydrates [13]. Thus, it is preferable to prepare flame-retardant thermoplastics directly from the plant biomass, based on the effort, time, cost, and yield. Therefore, in this study, flame retardancy and thermoplasticity were added to the plant biomass without separation and purification. The poor solubility of the plant biomass is herein problematic. The plasticization of wood through heterogeneous reactions has been reported [14, 15], and a homogeneous reaction is preferable for obtaining homogeneous plant-derived plastics because plant cell walls have a heterogeneous hierarchical structure. Specific solvents, such as dimethyl sulfoxide (DMSO)/LiCl and DMSO/tetrabutylammonium fluoride, dissolve wood; however, they require ball-milling as a pretreatment [16, 17]. On the other hand, ionic liquids have been reported to dissolve plant biomass without any pretreatment [18,19,20,21]. Therefore, it is expected that the hydroxy groups of the plant biomass can be substituted with [C2mim][(MeO)(H)PO2] as well as cellulose, i.e., the mixing of [C2mim][(MeO)(H)PO2] and plant biomass while heating, and the products can be used as flame-retardant thermoplastics.
The low thermal fluidity of plant biomass is a concern when making flame-retardant thermoplastics from plant biomass. Plant biomass does not exhibit fluidity, even at high temperatures, because of three factors. The first factor is the high crystallinity of cellulose [22]. The softening point can be decreased by destroying the hydrogen bonding network between the cellulose chains because of the high hydrogen bonding ability of the ionic liquids [9]. Furthermore, the softening point can also be decreased by substituting the hydroxy groups with [(MeO)(H)PO2] anions because the substitution decreases the number of hydroxy groups. The effectiveness of this strategy has been proven because IL-cellulose shows fluidity at an intermediate temperature [6]. The second factor is that a certain amount of lignin in the plant biomass chemically bonds with hemicellulose and forms a large macromolecule, referred to as the lignin carbohydrate complex (LCC) [23]. The third factor is the high branching of lignin [24, 25]. Regarding these factors, we have reported that the LCC and lignin are partially cleaved in [C2mim][(MeO)(H)PO2] and a similar ionic liquid at ~120 °C [26, 27]. Because substitution with [C2mim][(MeO)(H)PO2] is conducted at the high temperature of 160 °C, the partial cleavage of LCC and lignin could simultaneously occur. In this study, the direct preparation of flame-retardant thermoplastics was attempted by adducting [C2mim][(MeO)(H)PO2] to the plant biomass by mixing at 160 °C based on these hypotheses. This method further enables the development of flame-retardant plant thermoplastics with a low substitution ratio because [C2mim][(MeO)(H)PO2] acts as a plasticizer and flame retardant, similar to IL-cellulose. This method makes room for further functionalization for molding and practical use, as mentioned above. To the best of our knowledge, this is the first study on the direct preparation of a flame-retardant thermoplastic from plant biomass without pretreatment.
Experimental procedure
Materials
Bagasse, Japanese cedar, and eucalyptus (Sanwa Ceruciron Co., Ltd., Yokkaichi, Japan) with a particle size of 250–500 μm were purchased and used. 1-ethyl-3-methylimidazolium acetate ([C2mim]OAc) (purity: ≥95%, Iolitec Ionic Liquids Technologies GmbH, Heilbronn, Germany) was purchased and used after drying. Dichloromethane, DMSO, and isopropenyl acetate (Sigma-Aldrich Co., LLC., St. Louis, MO, USA) were purchased and used as received. Acetone (Kanto Chemical Co. Inc., Tokyo, Japan) was purchased and used as received. [C2mim][(MeO)(H)PO2] (purity: ≥97%, Kanto Chemical Co. Inc., Tokyo, Japan) was purchased and used after drying. A dialysis membrane (MWCO 1000) (Funakoshi Co. Ltd., Tokyo, Japan) was purchased and used as received. Triethyl phosphate and vinyl decanoate (Tokyo Chemical Industry Co. Ltd., Tokyo, Japan) were purchased and used as received. Cellulose propionate (Scientific Polymer Products, Inc., New York, USA) was purchased and used as received. Filter paper (No. 5 C) derived from cellulose (Advantec, Co., Ltd., Tokyo, Japan) was purchased and used as received.
Synthesis of [C2mim][(MeO)(H)PO2]-substituted plant biomass (IL-bagasse, IL-cedar, and IL-eucalyptus)
The introduction of [C2mim][(MeO)(H)PO2] to the plant biomass was conducted based on previous reports [6, 28]; however, [C2mim]OAc was added to improve the biomass solubility (a detailed explanation is provided in the results and discussion). Bagasse, Japanese cedar, and eucalyptus (0.5 g) were each added into [C2mim]OAc (5.0 g). The biomass was dissolved by heating at 120 °C for 2 h. Then, 5.0 g of [C2mim][(MeO)(H)PO2] was added and heated to 160 °C for 3 h under an Ar atmosphere. Water (20 mL) was added to the resulting samples, and the unreacted ionic liquids were removed by dialysis for 48 h (water was exchanged every 8 h) with 500 mL of water. After filtration, the filtrates were dried in vacuo (IL-bagasse: 0.28 g, IL-cedar: 0.26 g, and IL-eucalyptus: 0.26 g as final yields from 0.50 g of each raw biomass). The [C2mim][(MeO)(H)PO2]-substituted biomasses are referred to as IL-bagasse, IL-cedar, and IL-eucalyptus in this study.
The structures of the IL-bagasse, IL-cedar, and IL-eucalyptus were confirmed by 1H NMR in DMSO-d6 at room temperature (ECA-600, JEOL, Ltd., Tokyo, Japan), while the measurement was conducted using the soluble part, and FT-IR (NICOLETis10, Thermo Fisher Scientific, K.K., Tokyo, Japan). The removal of unreacted IL was also confirmed by 1H NMR. Signals attributed to unreacted [C2mim][(MeO)(H)PO2], especially sharp P-H and P-CH3 signals, were not observed. The substitution ratios of the hydroxy groups of the plant biomass with [C2mim][(MeO)(H)PO2] were measured by 31P NMR (ECA-600, JEOL, Ltd., Tokyo, Japan). Triethyl phosphate was added as a standard for quantification. Each substitution ratio in terms of mol of IL-bagasse, IL-cedar, and IL-eucalyptus was estimated from the results of 31P NMR and the amount of hydroxy groups included in the plant biomass species. The amount of hydroxy groups in the plant biomass was calculated from the content of cellulose, hemicellulose, and lignin determined by the NREL method [29] and the number of hydroxy groups of each component (e.g., three hydroxy groups in one anhydroglucose unit). The hydroxy group content of lignin was 4.26 mmol/g, measured using a method described in previous literatures [6, 29]. Alkali lignin was used as the model lignin to estimate the hydroxy group content.
We tried gel permeation chromatography measurement of the samples to check for possible degradation of polymers, but the procedure was impossible, at least with our systems, because of the poor solubilities of the raw- and IL-biomasses. We were able to subject only IL-lignin to gel permeation chromatography, and the molecular weight was shifted to a higher molecular weight by the adduction of [C2mim][(MeO)(H)PO2] compared to that of unreacted alkali lignin (Fig. S1). We can say that the “lignin is still a polymer” but cannot describe the polysaccharides. Neither can we say that “alkali lignin does not decompose at all” because we conducted dialysis for purification, and alkali lignin is different from native lignin. The gel permeation chromatography (Prominence UFLC system, Shimadzu Co., Kyoto, Japan) was conducted with polystyrene standards using TSK gel α-M (Tosoh Co., Tokyo, Japan). LiBr (0.01 mol L−1) in dimethylformamide (HPLC grade, Kanto Chemicals Co., Inc., Tokyo, Japan) was used as an eluent (flow rate: 1.0 mL min−1) at 40 °C.
Synthesis of bagasse acetate decanoate
Bagasse acetate decanoate was synthesized for comparison with the IL-biomass samples. Two different types of acyl groups were introduced because mixing long- and short- alkyl-chains significantly decreases the apparent melting point. Bagasse was successively decanoylated and acetylated in [C2mim]OAc/DMSO, referring to the literature [30]. The synthetic procedures are briefly described below.
Bagasse (3.0 g), [C2mim]OAc (50 g), and DMSO (75 mL) were mixed under an Ar atmosphere. The obtained solution was stirred at 110 °C for 16 h. After cooling the solution to 80 °C, a small amount of vinyl decanoate (2.1 mL) and an excess amount of isopropenyl acetate (100 mL) were added to the solution in order, and each reaction was conducted for 30 min. The substitution ratio was 97% (acetyl group: 68%, decanoyl group: 29%).
Evaluation of thermoplasticity
To evaluate the thermoplasticity of the IL-biomass samples, the minimum film-forming temperature was used, defined as the temperature at which two overlapped sample films were pressed at 45 kN using a hot press (Imoto Machinery Co., Ltd., Kyoto, Japan) and could be molded into one sheet cleanly. The minimum film-forming temperature was investigated by increasing the temperature by 10 °C increments from 80 °C. The thermal decomposition temperature was evaluated using thermogravimetric analysis (TGA) (DTG-60AH, Shimadzu Co., Kyoto, Japan) under air (10 °C/min). Differential scanning calorimetry (DSC) (DSC 60A plus, Shimadzu Co., Kyoto, Japan) was conducted with a heating scan rate of 10 °C/min from 30 to 170 °C.
Evaluation of the flame retardancy
The quantitative flame-retardancy was evaluated by the residual weights in the TGA under nitrogen and air. In addition, the combustion behavior of the samples was observed using an alcohol lamp.
Results and discussion
Bagasse (grass), Japanese cedar (softwood), and eucalyptus (hardwood), which are known to be produced as waste in manufacturing processes, were used as the samples of plant biomass. The biomass samples used in this study were prepared simply by cutting/crushing with a mixer, without intense pretreatments such as ball-milling. These samples are similar to real waste such as roughly crushed bagasse and sawdust. The use of fine biomass waste has faced limitations, while large wood stuffs are used as architectural materials. [C2mim][(MeO)(H)PO2]-substituted plant biomass was prepared similarly to IL-lignin, as previously reported (lignin was dissolved in [C2mim][(MeO)(H)PO2] at 160 °C and reacted for 3 h) [6]. However, IL-bagasse could not be obtained because the bagasse was not sufficiently dissolved in [C2mim][(MeO)(H)PO2]. Herein, we focus on 1-ethyl-3-methylimidazolium acetate ([C2mim]OAc), which has a better dissolution ability for the plant biomass [31, 32]. Bagasse was stirred in [C2mim]OAc at 120 °C for 2 h, and a large percentage of the bagasse was dissolved based on visual confirmation. Then, [C2mim][(MeO)(H)PO2] was added as a co-solvent and reactant, which did not cause precipitation because of its intermediate dissolution ability, and reacted at 160 °C for 3 h. IL-cedar and IL-eucalyptus were prepared using the same method.
In the 1H NMR spectrum of a soluble part of the IL-bagasse, peaks derived from [C2mim] cations were observed at 1.37, 3.82, 4.16, 7.68, 7.77, and 9.23 ppm, while peaks derived from the [(MeO)(H)PO2] anions were observed at 6.01 and 6.99 ppm (Fig. S2). Furthermore, the signal attributed to the methyl group of the [(“Me”O)(H)PO2] anion (~3.2 ppm) was not observed in the spectrum of IL-bagasse. These results suggest that the [(MeO)(H)PO2] anion was covalently introduced into bagasse, accompanied by the removal of the methyl group of the [(“Me”O)(H)PO2] anion. IL-cedar and IL-eucalyptus were also confirmed to have been synthesized in addition to IL-bagasse (see Fig. S2). The introduction of [C2mim][(MeO)(H)PO2] into each plant biomass was also confirmed by FT-IR (Fig. S3). Peaks derived from the P–O–C bond at 821 cm−1, the P = O bond at 1211 cm−1, and the C = N bond at 1571 cm−1 were detected in the IL-bagasse, while these were not observed in the unreacted bagasse [33, 34]. Peaks derived from these bonds were also detected in IL-cedar and IL-eucalypts (see Fig. S3). The signals derived from P = O and C = N were observed at similar wavenumbers in [C2mim][(MeO)(H)PO2]; however, the signal from P–O–C was not observed at ~821 cm−1. The signal at 744 cm−1 may be the corresponding signal because P–O–“C” changed from the methyl group to the carbon atoms of the carbohydrates and lignin. These results indicated that [C2mim][(MeO)(H)PO2] was covalently introduced to each plant biomass.
The substitution ratios of the hydroxy groups of bagasse, cedar, and eucalyptus with [C2mim][(MeO)(H)PO2] were roughly estimated by 31P NMR (spectra are shown in Fig. S4). Their substitution ratios were 13, 8, and 10 mol%, respectively, against the number of hydroxy groups. Because the substitution ratios of IL-cellulose, IL-xylan, and IL-lignin were 33, 32, and 14 mol%, respectively [6], those of the IL-biomass samples were lower. This could be because of the lignin contained in the plant biomass and the lower concentration of [C2mim][(MeO)(H)PO2] owing to dilution by the [C2mim]OAc used to dissolve the biomass. From these results, IL-bagasse, IL-cedar, and IL-eucalyptus maintained 87, 92, and 90 mol% of the hydroxy groups, respectively, after derivatization. Therefore, there is potential for further functionalization.
The thermal properties of the samples regarding thermoplasticity were investigated (Table 1). In this study, the film formation ability by hot pressing was evaluated as the thermoplasticity. The minimum film-forming temperatures were determined using a hot press machine, and these values for IL-bagasse, IL-cedar, and IL-eucalyptus were 160, 140, and 140 °C. The resulting films shown in Fig. 2 were flexible. In addition, these films were brown in color owing to the lignin content but were transparent. It seems surprising that thermoplasticity was shown with only a 10 mol% substitution ratio. Here, as an example, Chen et al. reported that mulberry wood shows thermoplasticity at 150 °C with an ionic liquid addition (i.e., just mixing) of <10 wt% against the weight of the biomass [10]. The substitution ratios of the samples, IL-bagasse, IL-cedar, and IL-eucalyptus (13, 8, and 10 mol%, respectively), correspond to 28, 19, and 23 wt% of ionic liquid content, respectively. Therefore, it is not surprising that the IL-biomass samples showed film-forming temperatures at 140–160 °C. On the other hand, the minimum film-forming temperature was approximately the same as that of the sample prepared by only mixing with ionic liquid, although the ionic liquid contents in IL-biomass samples were ~2–3 times those of thermoplastics based on a biomass/IL mixture. This result is presumably due to the difference in the state of the ionic liquid, namely, free or fixed.
The composition ratios of cellulose, hemicellulose, and lignin contained in the bagasse, cedar, and eucalyptus used this research are listed in Table S1. The minimum film-forming temperatures of IL-bagasse, IL-cedar, and IL-eucalyptus were calculated as 133, 136, and 134 °C, respectively, from the ratio of the biopolymers and the individual minimum film-forming temperatures (IL-cellulose, IL-xylan, and IL-lignin: 100, 140, and 180 °C, respectively). The minimum film-forming temperature of all samples was over 140 °C, which was slightly higher than expected. The differences were attributed to the lower substitution ratio of the IL-biomass samples than that of IL-cellulose, IL-xylan, and IL-lignin. The composition of IL-cedar and IL-eucalyptus differed; however, both had a minimum film-forming temperature of 140 °C. This indicates that this method was not significantly affected by the biomass components. Conversely, the minimum film-forming temperature of IL-bagasse was higher than that of IL-eucalyptus, although the composition ratio of the biopolymer was approximately the same. Because the substitution ratio of IL-bagasse was higher than that of IL-eucalyptus, other factors such as the molecular weights of the components might have caused the slight difference. However, considering that the minimum film-forming temperature of the native biomass species is much higher than the thermal decomposition temperature, the difference between 140 °C and 160 °C does not seem large. To investigate further characteristics of the samples, the samples were subjected to DSC (Fig. S5). Some glass transition temperatures were observed, but it is difficult to clarify the relationships between the minimum film-forming temperatures and the glass transition temperatures because the biomasses are intrinsically mixtures of polymers.
Plant biomass plastics synthesized by a heterogeneous reaction have a melting point of 140–320 °C [14, 24, 25]. In addition, the minimum film-forming temperature of bagasse acetate decanoate (substitution ratio: 97%) was 120 °C. It is known that the thermoforming temperature decreases upon introducing a long alkyl chain and a short alkyl chain. Based on these results, the plant biomass, bagasse, cedar, and eucalyptus, which cannot normally form films, was able to form thin films after substituting [C2mim][(MeO)(H)PO2] for the hydroxy groups. In addition, these samples showed minimum film-forming temperatures close to that of bagasse acetate decanoate upon having a high substitution ratio.
The thermal decomposition temperatures of the samples (the temperature at which the residual weight is 95%) were determined by TGA under an air atmosphere. IL-bagasse, IL-cedar, and IL-eucalyptus showed thermal decomposition temperatures of 229, 234, and 232 °C, respectively (full TGA curves are shown in Fig. S6). The thermal decomposition temperatures were higher than the minimum film-forming temperatures (160, 140, and 140 °C), indicating their possible use as thermoplastics. The thermal decomposition temperatures of untreated cedar and eucalyptus were 245 and 260 °C, which confirmed that the substitution of [C2mim][(MeO)(H)PO2] decreased the thermal decomposition temperature by 11 and 28 °C, respectively. The decrease of the thermal decomposition temperature can be explained by the decomposition and char formation temperature of phosphorus compounds of ~230 °C. The thermal decomposition temperature of IL-bagasse showed a minimal change after substitution with [C2mim][(MeO)(H)PO2] (from 234 to 229 °C) because the thermal decomposition temperature of bagasse was near that of the phosphorus component. The same trend was observed when cellulose was derivatized (shifting from 313 to 230 °C). In contrast, the thermal decomposition temperature of bagasse acetate decanoate was 191 °C, indicating a higher thermal stability of the IL-biomass samples than that of typical biomass thermoplastics.
The thermal properties of the polymers regarding flame retardancy were measured (see Table 1). The residual weight of each sample was evaluated at 400 °C by TGA under an air atmosphere as an index of the flame-retardancy [35] (see Fig. S6). Phosphoric acid-type flame retardants degrade at ~230 °C, as mentioned above, creating polyphosphate layers as char when combusted, and the layers block oxygen and heat [35]. Thus, the residual weight at over 230 °C, which is the char formation ability, is an indicator of the flame retardancy. The residual weights of IL-bagasse, IL-cedar, and IL-eucalyptus at 400 °C were 56, 57, and 55%, respectively. The residual weights of untreated bagasse, cedar, and eucalyptus at 400 °C were 19, 26, and 21%, respectively. These suggest that the substitution of [C2mim][(MeO)(H)PO2] significantly increased the residual weight. On the other hand, the residual weight of bagasse acetate decanoate, a bagasse-based thermoplastic, was 22% and was approximately equal to that of the untreated biomass, indicating that it is not a flame-retardant material. However, the residual weight of bagasse acetate decanoate was higher than that of CP (12%) owing to the lignin. The flame retardancy of the IL-biomass samples was confirmed based on the TGA. The residual weight of a flame-retardant cellulose previously reported by Aoki et al. (i.e., a cellulose derivative prepared by adding a phosphoric acid-type flame retardant into CP using two steps, as mentioned in the introduction section) was ~30% at 400 °C [5]. In addition, the residual weight of flame-retardant sawdust (Scots pine sapwood) prepared by substitution with diethyl chlorophosphite in pyridine [35], although not a thermoplastic, was ~50% at 400 °C, and the flame retardancies of the IL-biomass samples were equivalent. The flame-retardant effect of [C2mim][(MeO)(H)PO2] substitution was evaluated based on the weight percent gain (WPG). The WPG of the flame-retardant sawdust produced using diethyl chlorophosphite was 32%, and the residual weight was 52%. In our sample, the WPG of IL-bagasse was 39%, and the residual weight was 56%. Based on these results, this method of substituting [C2mim][(MeO)(H)PO2] could sufficiently impart flame retardancy based on the WPG.
The residual weights of the IL-biomass samples were estimated from the residual weight of IL-cellulose, IL-xylan, and IL-lignin and the composition of the components in the biomass. The estimated residual weight was 58, 60, and 59% for IL-bagasse, IL-cedar, and IL-eucalyptus, respectively. The experimental values of the IL-biomass samples were similar to the estimated values (56, 57, and 55%, respectively). This indicates that the performance of [C2mim][(MeO)(H)PO2] in the IL-biomass samples was equivalent to or more than that in the IL-pure biopolymers when considering the substitution ratios. These results may also indicate that the flame retardancy of the IL-biomass samples can be presumed. Based on these results, this method simultaneously imparted thermoplasticity and flame retardancy directly to the biomass, while maintaining hydroxy groups and adding good flame retardancy equivalent to that of IL-pure biopolymers, which was higher than that of the flame-retardant cellulose thermoplastic previously reported [5]. In addition, this method was applicable to a broad range of biomass species, including grasses, softwoods, and hardwoods.
The TGA of each sample was also conducted under nitrogen (Fig. S7). The residual weights of IL-bagasse, IL-cedar, and IL-eucalyptus at 400 °C were 51, 55, and 52%, respectively, and were approximately equal to those in air (56, 57, and 55%, respectively). In addition, van Krevelen reported a rough linear relationship between the limited oxygen index (LOI) and the residual weight at 850 °C under nitrogen [36]. When this relation was applied to the samples, the corresponding LOI was approximately 0.31, 0.26, and 0.31 for IL-bagasse, IL-cedar, and IL-eucalyptus, respectively. Because materials with an LOI ≤ 0.26 are considered flammable materials [36], the IL-bagasse, and IL-eucalyptus samples are recognized as flame-retardant materials, while IL-cedar is marginal. The residual weight of bagasse acetate decanoate at 850 °C was 3%, and the corresponding LOI was 0.19, which suggests a similar flammability to that of cellulose (LOI: 0.19) [36].
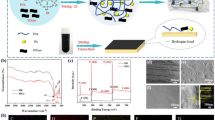
The samples were subjected to a brief fire test (Fig. 3 shows the photos of the IL-biomass samples after the fire test, and Supplemental movies exhibit the burning behaviors). When the CP film contacted the fire of an alcohol lamp, it burned completely, forming a slight char and dripping. Bagasse acetate decanoate also burned completely, forming a slight char (Fig. S8). For bagasse acetate decanoate, there was no dripping, and the burning speed was slower. This difference could be because of the lignin, which is flame-retardant. For the IL-biomass samples, combustion started; however, char layers instantly formed, and the fire was self-extinguished. This was caused by the flame-retardant effect of [C2mim][(MeO)(H)PO2] because the behaviors of the samples were consistent with those of phosphoric acid-type flame retardants. Therefore, thermoplasticity and firm flame retardancy were simultaneously imparted to grass, hardwood, and softwood by substituting with [C2mim][(MeO)(H)PO2].
The char layers formed on the samples were observed by scanning electron microscopy (Fig. S9). The surfaces of the char layers of the IL-biomass samples were smooth, and there were numerous cavities of ~10–30 µm. Some of the cavities were independent, indicating foaming during formation of the char layers. We have reported that [C2mim][(MeO)(H)PO2] acted as an intumescent-type flame retardant for cellulose, xylan, and lignin [6]. In this research, it has also been confirmed that [C2mim][(MeO)(H)PO2] acts as an intumescent-type flame retardant for plant biomass. Conversely, the char layers of bagasse acetate decanoate were rough, with no cavities of ~10–30 µm, although very small, continuous cavities (approximately 1 µm) were observed. Therefore, the introduction of [C2mim][(MeO)(H)PO2] to the plant biomass caused the generation of cavities of ~10 μm separated by smooth char layers when flamed, which enhanced the flame retardancy.
Thus, flame-retardant thermoplastics were developed from grass and wood biomasses with [C2mim][(MeO)(H)PO2] at a low substitution ratio (≤13%). The ability to enable thermoplasticity and flame-retardancy with low substitution was attributed to functionalization by only a single species, [C2mim][(MeO)(H)PO2]. This method produced flame-retardant plastics without requiring any extraction processes of single components, such as cellulose, or pretreatment processes of the biomass, such as ball-milling. This is a new proposal for the reuse of fine biomass waste, such as bagasse and sawdust.
Conclusion
Thermoplasticity and flame retardancy were simultaneously imparted to plant biomass bagasse, cedar, and eucalyptus by dissolution into a [C2mim][(MeO)(H)PO2]/[C2mim]OAc mixture and precipitation. During dissolution, the hydroxy groups in the biomass were substituted with [C2mim][(MeO)(H)PO2] with a substitution ratio of <14%, maintaining more than 86% of the hydroxy groups, thus demonstrating the potential for further functionalization. Furthermore, substitution with [C2mim][(MeO)(H)PO2] prevented bleeding out. The minimum film-forming temperatures of IL-bagasse, IL-cedar, and IL-eucalyptus were 160, 140, and 140 °C, respectively, which were similar to the corresponding value of bagasse acetate decanoate (120 °C). The minimum film-forming temperatures of the IL-biomass samples were sufficiently lower than the thermal decomposition temperatures (229, 234, and 232 °C, respectively), and these materials could be used as thermoplastics. For quantitative evaluation of the flame retardancy, the residual weights of the IL-biomass samples at 400 °C under air were investigated. The residual weights of IL-bagasse, IL-cedar, and IL-eucalyptus were 56, 57, and 55%, respectively. These were much larger than those of the raw biomasses (~20%) and bagasse acetate decanoate (23%) and were equivalent to that of the flame-retardant (not thermoplastic) wood powder previously reported. In the burning test, while the CP and bagasse acetate decanoate were burned out, forming a slight char, the IL-biomass samples formed foamed char layers and then self-extinguished. The foamed char layers indicated that [C2mim][(MeO)(H)PO2] acted as a thermoplasticizer and intumescent-type flame retardant, which is a very effective flame retardant.
References
Edgar KJ, Buchanan CM, Debenham JS, Rundquist PA, Seiler BD, Shelton MC, et al. Advances in cellulose ester performance and application. Prog Polym Sci. 2001;26:1605–88.
Klemm D, Heublein B, Fink HP, Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed. 2005;44:3358–93.
Mekonnen T, Mussone P, Khalil H, Bressler D. Progress in bio-based plastics and plasticizing modifications. J Mater Chem A. 2013;1:13379–98.
Kosaka PM, Kawano Y, Petri HM, Fantini MCA, Petri DFS. Structure and properties of composites of polyethylene or maleated polyethylene and cellulose or cellulose esters. J Appl Polym Sci. 2007;103:402–11.
Aoki D, Nishio Y. Phosphorylated cellulose propionate derivatives as thermoplastic flame resistant/retardant materials: influence of regioselective phosphorylation on their thermal degradation behavior. Cellulose. 2010;17:963–76.
Nishita R, Kuroda K, Ota S, Endo T, Suzuki S, Ninomiya K, et al. Flame-retardant thermoplastics derived from plant cell wall polymers by single ionic liquid substitution. New J Chem. 2019;43:2057–64.
Fukaya Y, Hayashi K, Wada M, Ohno H. Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions. Green Chem. 2008;10:44–46.
Kuroda K, Fukaya Y, Ohno H. Direct HPILC analysis of cellulose depolymerisation in ionic liquids. Anal Methods. 2013;5:3172–6.
Wu J, Bai J, Xue Z, Liao Y, Zhou X, Xie X. Insight into glass transition of cellulose based on direct thermal processing after plasticization by ionic liquid. Cellulose. 2015;22:89–99.
Chen J, Chen X, Su M, Ye J, Hong J, Yang Z. Direct production of all-wood plastics by kneading in ionic liquids/DMSO. Chem Eng J. 2015;279:136–42.
Miyafuji H, Fujiwara Y. Fire resistance of wood treated with various ionic liquids. Holzforschung. 2013;67:787–93.
Kim JS, Lee YY, Kim TH. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol. 2016;199:42–48.
Chakar FS, Ragauskas AJ. Review of current and future softwood kraft lignin process chemistry. Ind Crops Prod. 2004;20:131–41.
Hassan ML, El-Wakil NA, Sefain MZ. Thermoplasticization of bagasse by cyanoethylation. J Appl Polym Sci. 2001;79:1965–78.
Lu X, Zhang MQ, Rong MZ, Shi G, Yang GC. All-plant fiber composites I. Unidirectional sisal fiber reinforced benzylated wood. Polym Compos. 2002;23:624–33.
Wang Z, Yokoyama T, Chang HM, Matsumoto Y. Dissolution of beech and spruce milled woods in LiCl/DMSO. Agric J Food Chem. 2009;57:6167–70.
Lu F, Ralph J. Non-degradative dissolution and acetylation of ball-milled plant cell walls: high-resolution solution-state NMR. Plant J. 2003;35:535–44.
Kilpeläinen I, Xie H, King A, Granstrom M, Heikkinen S, Argyropoulos DS. Dissolution of wood in ionic liquids. J Agric Food Chem. 2007;55:9142–8.
Fort DA, Remsing RC, Swatloski RP, Moyna P, Moyna G, Rogers RD. Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem. 2007;9:63–69.
Brandt A, Gräsvik J, Hallett JP, Welton T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013;15:550–83.
Chen MJ, Shi QS. Transforming sugarcane bagasse into bioplastics via homogeneous modification with phthalic anhydride in ionic liquid. ACS Sustain Chem Eng. 2015;3:2510–5.
Hon DN-S. Chemical modification of lignocellulosic materials. 1st ed. CRC Press; Boca Raton, U.S. 1995.
Salanti A, Zoia L, Tolppa EL, Orlandi M. Chromatographic detection of lignin–carbohydrate complexes in annual plants by derivatization in ionic liquid. Biomacromolecules. 2012;13:445–54.
Thiebaud S, Borredon ME. Solvent-free wood esterification with fatty acid chlorides. Bioresour Technol. 1995;52:169–73.
Shiraishi N, Yoshioka M. Plasticization of wood by acetylation with trifluoroacetic acid pretreatment. Sen’i Gakkaishi. 1986;42:84–93.
Kuroda K, Fukaya Y, Yamada T, Ohno H. Molecular weight distributions of polysaccharides and lignin extracted from plant biomass with a polar ionic liquid analysed without a derivatisation process. Anal Methods. 2015;7:1719–26.
Kuroda K, Kunimura H, Fukaya Y, Nakamura N, Ohno H. 1H NMR evaluation of polar and nondeuterated ionic liquids for selective extraction of cellulose and xylan from wheat bran. ACS Sustain Chem Eng. 2014;2:2204–10.
Vo HT, Kim YJ, Jeon EH, Kim CS, Kim HS, Lee H. Ionic-liquid-derived, water-soluble ionic cellulose. Chem Eur J. 2012;18:9019.
Sakai H, Kuroda K, Tsukegi T, Ogoshi T, Ninomiya K, Takahashi K. Butylated lignin as a compatibilizing agent for polypropylene–based carbon fiber-reinforced plastics. Polym J. 2018;50:997–1002.
Suzuki S, Shibata Y, Hirose D, Endo T, Ninomiya K, Kakuchi R, et al. Cellulose triacetate synthesis via one-pot organocatalytic transesterification and delignification of pretreated bagasse. RSC Adv. 2018;8:21768–76.
Sun N, Rahman M, Qin Y, Maxim ML, Rodríguez H, Rogers RD. Complete dissolution and partial delignification of wood in ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009;11:646–55.
Li W, Sun N, Stoner B, Jiang X, Lu X, Rogers RD. Rapid dissolution of lignocellulosic biomass in ionic liquids using temperatures above the glass transition of lignin. Green Chem. 2001;13:2038–47.
Suflet DM, Chitanu GC, Popa VI. Phosphorylation of polysaccharides: New results on synthesis and characterisation of phosphorylated cellulose. React Funct Polym. 2006;66:1240–9.
Campbell JLE, Johnson KE, Torkelson JR. Infrared and variable-temperature 1H-NMR investigations of ambient-temperature ionic liquids prepared by reaction of HCl with 1-ethyl-3-methyl-1H-imidazolium chloride. Inorg Chem. 1994;33:3340–5.
Stevens R, van Es DS, Bezemer R, Kranenbarg A. The structure–activity relationship of fire retardant phosphorus compounds in wood. Polym Degrad Stab. 2006;91:832–41.
van Krevelen DW. Some basic aspects of flame resistance of polymeric materials. Polymer. 1975;16:615–20.
Acknowledgements
This research was supported in part by the COI program “Construction of next-generation infrastructure using innovative materials–Realization of a safe and secure society that can coexist with the Earth for centuries”, which is supported by the Ministry of Education, Culture, Sports, Science and Technology-Japan (MEXT) and the Japan Science and Technology (JST), the Advanced Low-Carbon Technology Research and Development Program (ALCA, No. JPMJAL1104) and the Cross-ministerial Strategic Innovation Promotion Program (SIP) of JST. This study was also partly supported by KAKENHI (18K14281 from the Japan Society for the Promotion of Science) and the Leading Initiative for Excellent Young Researchers (from MEXT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nishita, R., Kuroda, K., Suzuki, S. et al. Flame-retardant plant thermoplastics directly prepared by single ionic liquid substitution. Polym J 51, 781–789 (2019). https://doi.org/10.1038/s41428-019-0195-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-019-0195-2
This article is cited by
-
Latest advances in ionic liquids promoted synthesis and application of advanced biomass materials
Frontiers of Chemical Science and Engineering (2023)
-
An overview of regenerable wood-based composites: preparation and applications for flame retardancy, enhanced mechanical properties, biomimicry, and transparency energy saving
Advanced Composites and Hybrid Materials (2022)