Abstract
The stimuli-responsive color-changing properties of functional polymers are applied to the development of sensing and imaging devices. Tuning the stimuli responsivity is required for a wide range of applications. Polydiacetylene (PDA) derivatives show color changes with the application of external stimuli, such as heat and mechanical stress. Our group has focused on the layered crystal structure of PDA and its intercalation chemistry. The original layered PDA shows an irreversible color transition from blue to red upon heating to the threshold temperature. Here, we found that alkyldiamine-intercalated PDA possessed different color-changing properties, such as a higher color transition temperature and a temperature-dependent and reversible color change, than the original PDA. As the alkyl-chain length increased, the color-changing behavior gradually converted from the irreversible color transition type to the reversible temperature-dependent one. Since the intercalated diamine had a stabilizing effect on the layered structure, the stimuli responsivity varied according to the type of diamine. The results suggest that the stimuli-responsive color-changing properties of layered PDA can be finely and systematically tuned by the intercalation of organic guests.
Similar content being viewed by others
Introduction
Layered nanostructures are found in a variety of organic and inorganic materials [1,2,3]. Intercalation and exfoliation chemistries have been thoroughly studied in inorganic layered materials [4,5,6,7,8,9,10,11,12,13]. The intercalation of guests facilitates the generation of functional composite materials [14,15,16,17,18,19,20,21]. Organic layered materials have both intercalation and dynamic properties that, respectively, originate from the layered structure and the presence of organic molecules [22,23,24,25,26,27,28,29,30,31]. The characteristic structures and properties can be effectively applied to the development of functional nanomaterials. In the present work, we focused on tuning the stimuli-responsive color-changing properties of a crystalline layered organic polymer through the intercalation of guests in the interlayer space.
Layered polydiacetylene (PDA) shows stimuli-responsive color-changing properties [32,33,34,35,36]. A variety of diacetylene (DA) monomers, such as 10,12-pentacosadiynoic acid (PCDA), form a layered crystal structure (Fig. 1a). Topochemical polymerization proceeds by irradiation with light when the distance between the DA moieties is shorter than 0.5 nm [25, 37, 38]. PDA forms without changes in the layered crystal structure. External stimuli induce torsion of the PDA main chain through the motion of the alkyl side chains. The shortening of the effective conjugation length changes the color from blue to red. In previous reports [39,40,41,42,43,44], the molecular design and synthesis of DA derivatives was studied to control the stimuli-responsive color-changing properties of PDA, such as the color, responsivity, and reversibility. PCDA derivatives with a variety of functional groups instead of the carboxy group were synthesized to control the stimuli responsivity of the topochemically polymerized PDA [39,40,41,42,43,44]. These facts imply that the stability of the layered structure to external stimuli induces changes in the stimuli responsivity. If guest molecules intercalate into the interlayer space, the stimuli responsivity can be changed. In fact, the intercalation of guest molecules into layered PDA induced a color change because the intercalation itself acted as the external stimulus [45, 46]. In these previous works [45, 46], the intercalation of the guest into the interlayer space caused large structural changes in the layered PDA through the motion of the alkyl side chains and the torsion of the PDA main chain. The color change occurred due to shortening of the conjugation length. Therefore, a new approach is needed to control the stimuli responsivity by intercalation.
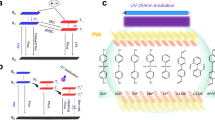
Schematic illustration of diamine-intercalated layered polydiacetylene (PDA) and its temperature-responsive color-changing properties. a Layered crystal structure of 10, 12-pentacosadiynoic acid (PCDA) molecules. b Layered PDA after the intercalation of guest diamines and subsequent topochemical polymerization. c Two types of color-changing behavior with heating and cooling that depend on the stabilization effect: irreversible transition (upper) and reversible temperature-dependent color change (lower). (Color figure online)
Our group has developed a new intercalation approach for tuning the stimuli-responsive color-changing properties [47,48,49,50,51,52]. Guest ions and molecules were intercalated into the layered structure of monomeric PCDA crystals before polymerization (Fig. 1b). When the metal cations intercalated into the interlayer space consisting of carboxy groups, the color-changing properties under heating, such as the color, responsivity, and reversibility, differed depending on the guest ions [47, 48, 50, 51]. The intercalated cations coordinate to the carboxy groups in the interlayer space. The stability of the layered structure to external stimuli is controlled by the intercalated guests. The stimuli-responsive color-changing properties were classified into two types: an irreversible color transition and reversible temperature-responsive color change (Fig. 1c) [48, 50]. The PDA host without an intercalated guest showed an irreversible color transition under heating to a certain temperature. When guests, such as nickel ions, intercalated into the layered PDA, the same color-changing properties were observed (upper panels in Fig. 1c). The color transition temperature was changed by changing the type of intercalated metal ions. When other guest ions, such as zinc ions, intercalated, the color gradually varied from blue to purple and then red based on the temperature of heating (lower panels in Fig. 1c). In addition, the red color was reverted to the original blue through cooling. The results suggest that the metal ions in the interlayer space change the stability of the layered structure to external stimuli. However, the relationship between the guest metal ions and the color-changing properties was not understood in previous works [47, 48]. In addition, the factors that determine these two types of color-changing behavior were not fully understood in previous works. If similar tuning of the color-changing properties is achieved with organic guest molecules, the relationship between the guests and the properties can be systematically studied according to the type of intercalated organic guest. Recently, we reported that alkylamine-intercalated layered PDA also showed tunable stimuli-responsive color-changing properties [52]. In the present study, we found that the alkyldiamine-intercalated PDA showed reversible temperature-dependent color-changing properties (Fig. 1c). The color-changing behavior gradually varied from the irreversible transition type corresponding to the original PDA to the reversible and temperature-dependent type with an increase in the alkyl-chain length of the intercalated diamines. In contrast, such a variation in the color-changing behavior was not observed for the PDA intercalated by normal alkylamine. These results suggest that the stabilization of the organic layered structure by the alkyldiamines plays an important role in the variation in the color-changing properties.
Experimental procedure
Synthesis of PDA with intercalated alkyldiamine
The experimental method was referenced to that in our previous report [52]. A commercial powder of 10,12-pentacosadiynoic acid (PCDA, TCI, Tokyo, Japan, 97.0%) was used as the monomeric host precursor crystal. The PCDA powder, typically 0.05 g, was dispersed in 20 cm3 of an aqueous solution containing the alkyldiamines (C n -(NH2)2, n = 4, 6, 8, 12) and 1 cm3 of tetrahydrofuran (THF, Kanto Tokyo, Japan, 99.5%). The following alkyldiamines were used as guests for the intercalation: 1,4-diaminobutane (C4-(NH2)2, NH2C4H8NH2, TCI, 98.0%), 1,6-diaminohexane (C6-(NH2)2, NH2C6H12NH2, Wako chemical, Osaka, Japan, 95.0%), 1,8-diaminooctane (C8-(NH2)2, NH2C8H16NH2, TCI, 98.0%), and 1,12-diaminododecane (C12-(NH2)2, NH2C12H24NH2, TCI, 98.0%). In addition, 4-(aminomethyl)benzylamine (Ph-(CH2NH2)2, NH2CH2C6H4CH2NH2, TCI, 98.0%) was used for the intercalation. A normal alkylamine, dodecylamine (C12-NH2, C12H25NH2, TCI, 97.0%), was used as the reference. The molar ratio of the amine to PCDA (Ramine/PCDA) was set at Ramine/PCDA = 2. The aqueous dispersion containing PCDA and the amines was maintained for 3 days at 25 °C under stirring. The resultant precipitate was collected by filtration and then washed with water/THF (95/5 by volume). In addition, water/ethanol (60/40 by volume) was used in the washing step when the guest diamine was in the solid-state at room temperature. Then, the powder was dried under vacuum for 10 h. The polymerization of the C n -(NH2)2- and Ph-(CH2NH2)2-intercalated PCDA samples was performed by irradiation with UV light (Ushio, Tokyo, Japan, xenon lamp UXL-500SX2, 500W) with a cutoff filter for visible light (Schott, Mainz, Germany UG-11) until the sample turned blue.
Structural characterization
The interlayer distance was measured by X-ray diffraction (XRD) with Cu-Kα radiation (XRD, Bruker, Yokohama, Kanagawa, D8-Advance). The state of the interlayer carboxy groups was analyzed by Fourier transform infrared spectroscopy (FT-IR, Jasco, Tokyo, Japan, FT/IR-4200). The powdered samples were prepared by the KBr method. The composition of the PCDA host and amine guest was analyzed by CHN elemental analysis.
Characterization of the temperature-responsive color-changing properties
Powdered samples of PDA and the amine-intercalated PDA were transferred to a stainless sample plate with a groove 5 × 7.5 mm in size and 1 mm in depth. The plate was placed on a temperature-controlled stage. The samples were heated to a certain temperature (T °C), held for 5 min, and then cooled to 25 °C. This operation, including the heating and subsequent cooling, was repeated with changes in the heating temperature from T = 30 °C to 170 °C in 5 °C increments. Photographs were taken for quantitative analysis of the color. The red color of the samples was characterized by the x-value on the basis of the image analysis (ITU-R BT.709, an international standard) [50,51,52,53].
Results
Formation of layered composites through intercalation and topochemical polymerization
The detailed structural characterization of C n -(NH2)2-intercalated PCDA (PCDA-C n -(NH2)2, n = 4, 6, 8, 12) was performed in our previous report [52]. Here, we show the structure of the new Ph-(CH2NH2)2-intercalated PCDA (PCDA-Ph-(CH2NH2)2) (Fig. 2). The original PCDA had a layered crystal structure with an interlayer distance (d0) of 4.78 nm (Figs. 1a and 2a). The peaks characteristic of the layered crystal structure were observed in the XRD pattern (pattern (i) in Fig. 2a). The diffraction peaks observed at 2θ = 1.85°, 3.68°, 5.56°, 7.42°, 9.31°, and 13.1° correspond to d0 / n (n = 1–6), respectively (filled circles in Fig. 2a). The intercalation of C n -(NH2)2 and Ph-(CH2NH2)2 caused an expansion of the d0 that depended on the molecular length (Fig. 2a) [52]. New diffraction peaks appeared at 2θ = 1.45°, 2.91°, 4.39°, 5.86°, 7.34°, 8.81°, 10.3°, 11.8°, and 13.2° after the intercalation of C12-(NH2)2 (triangles in Fig. 2a), although the peaks for the original PCDA were still visible. These peaks correspond to d0 / n (n = 1–9). The d0 shifted to 6.07 nm after the intercalation of C12-(NH2)2. PCDA-Ph-(CH2NH2)2 showed diffraction peaks at 2θ = 1.62°, 3.31°, and 4.95° (open circles in Fig. 2a). These peaks correspond to d0 / n (n = 1– 3) on the assumption that the expanded interlayer distance of PCDA-Ph-(CH2NH2)2 was d0 = 5.44 nm. The d0 value increased with an increase in the alkyl-chain length of C n -(NH2)2 (n = 4, 6, 8, 12) [52]. The tilt angle of the PCDA molecules and the intercalated amines was approximately 50° [52]. The original PCDA showed an absorption peak corresponding to the dimerized carboxy groups at approximately 1700 cm–1 in the FT-IR spectrum (Fig. 2b). After the intercalation of the guest amines, the peak for the carboxylate state was observed at approximately 1550 cm−1 (Fig. 2b). The FT-IR analysis indicates that the guest amine intercalated as the ammonium state into the interlayer space. The molar ratio of diamine to PCDA was estimated to be approximately 0.5 by CHN elemental analysis [52]. The results suggest that the intercalation of C n -(NH2)2 and Ph-(CH2NH2)2 into the PCDA monomer crystal was successful (Fig. 1b).
XRD patterns (a) and FT-IR spectra (b) of the original PCDA (i), PCDA-C12-(NH2)2 (ii), and PCDA-Ph-(CH2NH2)2 (iii). a The peaks marked with filled circles, open triangles, and open circles originated from the layered structure with d0 = 4.78 nm, d0 = 6.07 nm, and d0 = 5.44 nm, respectively. b The absorption bands marked with the white and black arrows correspond to the C=O stretching vibrations of the dimerized carboxy group and the carboxylate group, respectively. (Color figure online)
Temperature-responsive color-changing properties
The precursor layered composites of PCDA-C n -(NH2)2 and PCDA-Ph-(CH2NH2)2 were polymerized by irradiation with UV light. The sample color changed from colorless to blue. This color change is ascribed to the polymerization [32,33,34,35,36, 47,48,49,50,51,52]. Whereas the original PCDA had only monomeric diacetylene moieties, the topochemical polymerization reaction induced the formation of a conjugated main chain. Therefore, the resultant PDA exhibited a blue color. The layered composites of C n -(NH2)2- and Ph-(CH2NH2)2-intercalated PDA (PDA-C n -(NH2)2 and PDA-Ph-(CH2NH2)2) were formed. The stimuli-responsive color-changing properties were studied in heating and cooling cycles, as shown in Fig. 3a. The samples were heated to T °C, held for 5 min (upper panels in Fig. 3a–h), and then cooled to 25 °C (lower panels in Fig. 3a–h). The cycle of heating and cooling was repeated from T = 30 to 150 °C for PDA-C n -(NH2)2 and from T = 30 to 170 °C for PDA-Ph-(CH2NH2)2 at intervals of 5 °C (Fig. 3a–h). PDA without an interlayer guest showed a color change from blue to red at approximately 70 °C (upper panels in Fig. 3b). After the color changed to red under heating, the color was not recovered to the original blue under subsequent cooling (lower panels in Fig. 3b). The intercalation of C n -(NH2)2 and Ph-(CH2NH2)2 induced three characteristic differences in the color-changing behavior (Fig. 3c–g). The temperature of the color change from blue to red increased with an increase in the n of the intercalated C n -(NH2)2 (yellow arrows in Fig. 3b–g). The color-changing behavior from blue to red under heating varied from the irreversible transition type to the gradual temperature-dependent type (upper panels in Fig. 3b–g). Furthermore, the reversibility of the color change, namely, the recovery to the original blue color, was observed for PDA-C n -(NH2)2 and PDA-Ph-(CH2NH2)2 (yellow frames in Fig. 3b–g). In our previous work, these characteristic color-changing properties were observed for barium- and zinc-ion-intercalated PDA (PDA-Ba2+ and PDA-Zn2+) [48]. When the PDA intercalated with dodecylamine (C12-NH2) (PDA-(C12-NH2)) was used as a reference sample, these differences in the color-changing properties were not observed (Fig. 3h). The temperature of the color change based on the alkyl-chain length was different from that of the normal alkyl amines [52].
Temperature-responsive color-changing properties of amine-intercalated PDA. a Sample temperature with heating (upper panels) and cooling (lower panels). b–i Photographs of PDA (b), PDA-C4-(NH2)2 (c), PDA-C6-(NH2)2 (d), PDA-C8-(NH2)2 (e), PDA-C12-(NH2)2 (f), PDA-Ph-(CH2NH2)2 (g), and PDA-C12-NH2 (h) during the heating (upper panels) and subsequent cooling cycles (lower panels). The yellow arrows indicate the temperature of color change to red that can be detected by the naked eye. The yellow frames in the lower panels shows the reversible color change to the original blue. i, j Relationship between the sample temperature (T) and the x-value of the original PDA (i) and PDA-Ph-(CH2NH2)2 (j). The circles and squares correspond to the x-values that represent the intensity of the red color during the heating and cooling processes, respectively. ∆x, Ttrs, ∆T, and ∆xrev were defined as shown in panels i and j. (Color figure online)
The color-changing behavior was quantitatively analyzed through image analysis (Fig. 3i, j). Since the results of the spectroscopic analysis were consistent with those of the image analysis [50, 52], the facile image analysis was used in the present work. The intensity of the red color (x) was estimated from the photographs shown in Fig. 3b–h (See also: Experimental Section). The relationship between the sample temperature (T) and the x-value was observed during the heating and cooling processes (Fig. 3i, j). The overall color change from blue to red is represented by the difference between the initial and final x-values, namely, ∆x (Fig. 3i). The temperature range that achieved ∆x is defined as ∆T. The temperature of the color transition (Ttrs) is defined as the T that achieved 0.5∆x. The temperature-dependent gradual color-changing behavior during the heating process is represented by the slope of the curve, namely, ∆x/∆T. In the present study, ∆x/∆T was calculated from five points of x and T around Ttrs. The differences in the x-values of heating and cooling (∆xrev), namely, the differences of the x-values between the circles and squares in Fig. 3j, is interpreted as the reversibility of the color change.
The color transition temperature (Ttrs), temperature-dependent color-changing behavior (∆x/∆T), and reversibility (∆xrev) of PDA-C n -(NH2)2 and PDA-Ph-(CH2NH2)2 are summarized in Fig. 4. As a reference, the data for PDA-Ba2+ and the PDA-C12-NH2 are including for comparing the color-changing behavior [48, 50, 52]. The Ttrs increased with an increase in the alkyl-chain length of C n -(NH2)2 (triangles in Fig. 4a). PDA-Ph-(CH2NH2)2 and PDA-Ba2+ showed higher Ttrs values than PDA-C n -(NH2)2, whereas such a remarkable increase in the Ttrs was not observed for PDA-(C12-NH2). A smaller ∆x/∆T value indicates stronger temperature dependency of the color change during heating (circles in Fig. 4a). The ∆x/∆T value decreased with an increase in the alkyl-chain length of C n -(NH2)2. Smaller ∆x/∆T values were achieved for PDA-Ph-(CH2NH2)2 and PDA-Ba2+. In contrast, PDA-C12-NH2 showed a larger ∆x/∆T value. Although the PDA sample without an intercalated guest had a smaller ∆x/∆T value, such a remarkable temperature-dependent color change from blue to red was not observed, as shown in the photographs (Fig. 3b). The reversibility was characterized by the relationship between T and ∆xrev (Figs. 3j and 4b). Whereas diamine-intercalated PDA showed a larger ∆xrev, a remarkable ∆xrev was not observed for PDA and PDA-C12-NH2 (Fig. 4b).
Quantitative analysis of the color-changing properties. a ∆x/∆T and Ttrs of the PDA-related samples, representing the temperature dependency of the color and the color transition temperature, respectively. b Relationship between the temperature and ∆xrev, representing the reversibility of the color change. (Color figure online)
Discussion
The temperature-responsive color-changing properties were different for different types of intercalated guests (Figs. 3 and 4). The intercalation of diamines caused the emergence of temperature dependency and reversibility in the color-changing behavior as well as an increase in the Ttrs. These results suggest that intercalated guests, such as C n -(NH2)2, Ph-(CH2NH2)2, and Ba2+, enhance the stability of the layered structure to external stimuli by improving the interactions between PDA layers (Fig. 1b). The interlayer cation anchors the layers via electrostatic interactions. The intercalated C n -(NH2)2 and Ph-(CH2NH2)2 molecules have two terminal amines for anchoring the PDA layers via electrostatic interactions. An increase in the alkyl-chain length produces a stronger stabilization effect because the van der Waals interactions between adjacent diamine molecules enhances the stabilization effect. In contrast, such a strong stabilization effect is not achieved by the intercalation of C12-NH2, which gives a bilayer arrangement in the interlayer space. In our previous studies, metal-ion-intercalated PDA showed different color-changing properties that depended on the type of metal ions [48]. However, the influence of the metal ions on the color-changing properties was not fully studied. The present results suggest that the stronger stabilization effect directs the variations in the color-changing properties (Fig. 4).
Layered PDA showed a color transition at Ttrs without the temperature-dependent behavior and reversibility (Fig. 3b). Heating induces the thermal motion of the alkyl side chains of the layered PDA. The torsion of the PDA main chain causes the color change by shortening the conjugation length. Similar color change mechanisms have been reported in previous studies [54, 55]. It is inferred that the original conformation of layered PDA is stable. If the conformation is slightly changed by increasing the temperature, the conformation with torsion of the conjugated main chain is unstable. Therefore, the original conformation and color is preserved below the Ttrs. Since heating above the Ttrs induces large conformational changes originating from the thermal motion of the alkyl side chains, the color changes to red. When the layered structure is stabilized, the torsional conformations are also stabilized. Metastable torsional conformations are achieved during heating. Therefore, a gradual color change from blue to red with intermediate colors was observed during the heating process (Fig. 3). The stabilization effect is supported by the increase in the Ttrs (Fig. 4a). The stronger stabilization effect creates more remarkable temperature-dependent behavior, which is represented by ∆x/∆T (Fig. 4). The reversibility of the color change is also achieved because the metastable torsional conformations return to the original stable conformation under cooling. However, temperatures higher than the Ttrs causes color changes through large conformational changes. Recently, the importance of the metastable states in stimuli-responsive materials has been reported to induce color and fluorescence changes [56,57,58]. The present work implies that metastable states of the layered PDA are formed by the intercalation of guests.
Conclusions
The stimuli-responsive color-changing properties of layered PDA were varied by the intercalation of guest amines. Layered PDA showed an irreversible color transition from blue to red at the threshold temperature. The intercalation of diamines, such as C n -(NH2)2 and Ph-(CH2NH2)2, caused the emergence of temperature-dependent and reversible color-changing properties as well as an increase in the color transition temperature. In contrast, such color-changing properties were not observed for PDA intercalated with normal alkylamine. The intercalated diamines had a stabilization effect on the organic layered structure. Since metastable conformations formed in PDA-(C n -(NH2)2) and PDA-(Ph-(CH2NH2)2), temperature-dependent and reversible color-changing properties were achieved. These results suggest that the intercalation chemistry of the organic layered compounds has potential for tuning their dynamic properties.
References
Mallouk TE, Gavin J. Molecular recognition in lamellar solids and thin films. Acc Chem Res. 1998;31:209–17.
Ariga K, Ji Q, Hill JP, Bando Y, Aono M. Forming nanomaterials as layered functional structures toward materials nanoarchitectonics. NPG Asia Mater. 2012;4:e17.
Ozin GA. Nanochemistry: Synthesis in diminishing dimensions. Adv Mater. 1992;4:612–49.
Whittingham MS, Jacobson AJ, Eds. Intercalation chemistry. New York: Academic Press; 1982.
Schollhӧrn R. Intercalation systems as nanostructured functional materials. Chem Mater. 1996;8:1747–57.
Nakato T, Miaymoto N. Liquid crystalline behavior and related properties of colloidal systems of inorganic oxide nanosheets. Materials. 2009;2:1734–61.
Schaak RE, Mallouk TE. Perovskites by design: A toolbox of solid-state reactions. Chem Mater. 2002;14:1455–71.
Ma R, Sasaki T. Nanosheets of oxides and hydroxides: Ultimate 2D charge-bearing functional crystallites. Adv Mater. 2010;22: 5082–104.
Novoselov KS. Graphene: materials in the Flatland. Angew Chem Int Ed. 2011;50:6986–7002.
Wang Q, O’Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev. 2012;112:4124–55.
Rao CNR, Matte HSSR, Maitra U. Graphene analogues of inorganic layered materials. Angew Chem Int Ed. 2013;52:13162–85.
Nicolosi V, Chhowalla M, Kanatzidis MG, Strano MS, Coleman JN. Liquid exfoliation of layered materials. Science. 2013;340: 1226419.
Osada M, Sasaki T. Two-dimensional dielectric nanosheets: Novel nanoelectronics from nanocrystal building blocks. Adv Mater. 2012;24:210–28.
Ogawa M, Kuroda K. Photofunctions of intercalation compounds. Chem Rev. 1995;95:399–438.
Ogawa M, Kuroda K. Preparation of inorganic-organic nanocomposites through intercalation of organoammonium ions into layered silicates. Bull Chem Soc Jpn. 1997;70:2593–618.
Ruiz-Hitzky E, Darder M, Aranda P. Functional biopolymer nanocomposites based on layered solids. J Mater Chem. 2005;15: 3650–62.
Ogawa M, Saito K, Sohmiya M. A controlled spatial distribution of functional units in the two dimensional nanospace of layered silicates and titanates. Dalton Trans. 2014;43:10340–54.
Okada A, Usuki A. Twenty years of polymer-clay nanocomposites. Macromol Mater Eng. 2006;291:1449–76.
Kato M, Usuki A, Hasegawa N, Okamoto H, Kawasumi M. Development and applications of polyolefin– and rubber–clay nanocomposites. Polym J. 2011;43:583–93.
Osada M, Sasaki T. Nanosheet architectonics: a hierarchically structured assembly for tailored fusion materials. Polym J. 2015;47:89–98.
Haraguchi K. Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym J. 2011;43:223–41.
Kato T, Mizoshita N, Kishimoto K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew Chem Int Ed. 2006;45:38–68.
Ariga K, Yamauchi Y, Rydzek G, Ji Q, Yonamine Y, Wu KCW, Hill JP. Layer-by-layer nanoarchitectonics: Invention, innovation, and evolution. Chem Lett. 2014;43:36–68.
Coleman AW, Bott SG, Morley SD, Means CM, Robinson KD, Zhang H, Atwood JL. Novel layer structure of sodium calix[4]arenesulfonate complexes–a class of organic clay mimetics? Angew Chem Int Ed. 1988;27:1361–2.
Matsumoto A. Polymer structure control based on crystal engineering for materials design. Polym J. 2003;35:93–121.
Ohtake T, Ito K, Nishina N, Kihara H, Ohno H, Kato T. Liquid-crystalline complexes of a lithium salt with twin oligomers containing oxyethylene spacers. Approach anisotropic Ion Conduct Polym J. 1999;31:1155–8.
Kishimoto K, Yoshio M, Mukai T, Yoshizawa M, Ohno H, Kato T. Nanostructured anisotropic ion-conductive films. J Am Chem Soc. 2003;125:3196–7.
Högberg D, Soberats B, Yatagai R, Uchida S, Yoshio M, Kloo L, Segawa H, Kato T. Liquid-crystalline dye-sensitized solar cells: Design of two-dimensional molecular assemblies for efficient ion transport and thermal stability. Chem Mater. 2016;28:6493–6500.
Sakuda J, Yoshio M, Ichikawa T, Ohno H, Kato T. 2D Assemblies of ionic liquid crystals based on imidazolium moieties: Formation of ion-conductive layers. New J Chem. 2015;39: 4471–7.
Chikada M, Sada K, Miyata M. Intercalation and polymerization in chenodeoxycholic acid channels with retation of a crystalline state. Polym J. 1999;31:1061–4.
Matsumoto A, Fujioka A, Kunisue T. Organic intercalation of unsaturated amines into layered polymer crystals and solid-state photoreactivity of the guest molecules in constrained interlayers. Polym J. 2003;35:652–61.
Carpick RW, Sasaki DY, Marcus MS, Eriksson, Burns MA. Polydiacetylene films: a review of recent investigations into chromogenic transitions and nanomechanical properties. A. R. J Phys Condens Matter. 2004;16:R679.
Ahn DJ, Kim JM. Fluorogenic polydiacetylene supramolecules: Immobilization, micropatterning, and application to label-free chemosensors. Acc Chem Res. 2008;41:805–16.
Sun X, Chen T, Huang S, Li L, Peng H. Chromatic polydiacetylene with novel sensitivity. Chem Soc Rev. 2010;39: 4244–57.
Yarimaga O, Jaworski J, Yoon B, Kim JM. Polydiacetylenes: supramolecular smart materials with a structural hierarchy for sensing, imaging and display applications. Chem Commun. 2012;48:2469–85.
Jelinek R, Ritenberg M. Polydiacetylenes–Recent molecular advances and applications. RSC Adv. 2013;3:21192–201.
Wegner G. Topochemical polymerization of monomers with conjugated triple bonds. Die Makromol Chem. 1972;154:35–48.
Ogawa T. Diacetylenes in polymeric systems. Prog Polym Sci. 1995;20:943–85.
Lee S, Kim JM. r-Cyclodextrin: A molecule for testing colorimetric reversibility of polydiacetylene supramolecules. Macromolecules. 2007;40:9201–4.
Dei S, Matsumoto M, Matsumoto A. Thermochromism of polydiacetylenes in the solid state and in solution by the self-organization of polymer chains containing no polar group. Macromolecules. 2008;41:2467–73.
Ampornpun S, Montha S, Tumcharern G, Vchirawongkwin V, Sukwattanasinitt M, Wacharasindhu S. Odd−even and hydrophobicity effects of diacetylene alkyl chains on thermochromic reversibility of symmetrical and unsymmetrical diyndiamide polydiacetylenes. Macromolecules. 2012;45:9038–45.
Tanioku C, Matsukawa K, Matsumoto A. Thermochromism and structural change in polydiacetylenes including carboxy and 4-carboxyphenyl groups as the intermolecular hydrogen bond linkages in the side chain. ACS Appl Mater Interface. 2013;5:940–8.
Park IS, Park HJ, Kim JM. A soluble, low-temperature thermochromic and chemically reactive polydiacetylene. ACS Appl Mater Interface. 2013;5:8805–12.
Park IS, Park HJ, Jeong W, Nam J, Kang Y, Shin K, Chung H, Kim JM. Low temperature thermochromic polydiacetylenes: Design, colorimetric properties, and nanofiber formation. Macromolecules. 2016;49:1270–8.
Shimogaki T, Matsumoto A. Structural and chromatic changes of host polydiacetylene crystals during intercalation with guest alkylamines. Macromolecules. 2011;44:3323–7.
Yoon B, Jaworski J, Kim JM. Size-dependent intercalation of alkylamines within polydiacetylene supramolecules. Supramol Chem. 2013;25:54–59.
Okaniwa M, Oaki Y, Kaneko S, Ishida K, Maki H, Imai H. Advanced biomimetic approach for crystal growth in nonaqueous media: Morphology and orientation control of pentacosadiynoic acid and applications. Chem Mater. 2015;27:2627–32.
Okaniwa M, Oaki Y, Imai H. Intercalation-induced tunable stimuli-responsive color-change properties of crystalline organic layered compound. Adv Funct Mater. 2016;26:3463–71.
Ishijima Y, Okaniwa M, Oaki Y, Imai. Two exfoliation approaches for organic layered compounds: hydrophilic and hydrophobic polydiacetylene nanosheets. Chem Sci. 2017;8:647–53.
Takeuchi M, Imai H, Oaki Y. Real-time imaging of 2D and 3D temperature distribution: coating of metal-ion-intercalated organic layered composites with tunable stimuli-responsive properties. ACS Appl Mater Interfaces. 2017;9:16546–52.
Takeuchi M, Imai H, Oaki Y. Effects of intercalation rate on layered crystal structures and stimuli-responsive color-change properties of polydiacetylene. J Mater Chem C. 2017;5:8250–5.
Ishijima Y, Imai H, Oaki Y. Tunable mechano-responsive color-change properties of organic layered material by intercalation. Chem. 2017;3:509–21.
Reinhard E, Heidrich W, Debevec P, Pattanaik S, Ward G, Myszkowski K. High dynamic range imaging: Acquisition, display, and image-based lighting. 2nd ed, Ch. 2. Saint Louis: Elsevier Science; 2010, p. 35.
Sysoiev D, Fedoseev A, Kim Y, Exner TE, Boneberg J, Huhn T, Leiderer P, Scheer E, Groth U, Steiner UE. Synthesis and photoswitching studies of difurylperfluorocyclopentenes with extended π-systems. Chem Eur J. 2011;17:6663–72.
Ito S, Ando M, Nomura A, Morita N, Kabuto C, Mukai H, Ohta K, Kawakami J, Yoshizawa A, Tajiri A. Synthesis and properties of hexakis(6-octyl-2-azulenyl)benzene as a multielectron redox system with liquid crystalline behavior. J Org Chem. 2005;70:3939–49.
Sagara Y, Kato T. A Stimuli-responsive luminescent liquid crystals: Change of photoluminescent colors triggered by a shear-induced phase transition. Angew Chem Int Ed. 2008;47:5175–8.
Sagara Y, Yamane S, Mitani M, Weder C, Kato T. Mechanoresponsive luminescent molecular assemblies: An emerging class of materials. Adv Mater. 2016;28:1073–95.
Yagai, S, Okamura, S, Nakano, Y, Yamauchi, M, Kishikawa, K, Karatsu, T, Kitamura, A, Ueno, A, Kuzuhara, D, Yamada, H, Seki, T & Ito, H. Design amphiphilic dipolar π-systems for stimuli-responsive luminescent materials using metastable states. Nat. Commun. 2014;5:4013.
Acknowledgements
This work was partially supported by the Iketani Science and Technology Foundation (YO) and the Izumi Science and Technology Foundation (YO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Oaki, Y., Ishijima, Y. & Imai, H. Emergence of temperature-dependent and reversible color-changing properties by the stabilization of layered polydiacetylene through intercalation. Polym J 50, 319–326 (2018). https://doi.org/10.1038/s41428-017-0018-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-017-0018-2
This article is cited by
-
PJ ZEON Award for outstanding papers in Polymer Journal 2018
Polymer Journal (2019)