Abstract
The customized design of micro-/nanomotors represents one of the main research topics in the field of micro-/nanomotors; however, the effects of different crystal facets on micromotor movement are often neglected. In this work, self-propelled amorphous, cubic, and tetrahedral Ag3PO4 particles were synthetized using a scalable precipitation method. Their programmable morphologies exhibited different motion properties under fuel-free and surfactant-free conditions and visible light irradiation. Differences in these motion properties were observed according to morphology and correlated with photocatalytic activity. Moreover, Ag3PO4 micromotors are inherently fluorescent, which allows fluorescence-based tracking. Furthermore, bacterial biofilms represent a major concern in modern society since most of them are antibiotic resistant. The as-prepared self-propelled particles exhibited morphologically dependent antibiofilm activities toward gram-positive and gram-negative bacteria. The enhanced diffusion of the particles promoted biofilm removal in comparison with static control experiments, realizing the possibility of a new class of light-driven biofilm-eradicating micromotors that do not require the use of both H2O2 and UV light.
Similar content being viewed by others
Introduction
Light-driven nano-/micromotors have received great attention in recent years due to their interesting advantages compared to catalytic micromotors. The use of light to drive motion allows remote action in their movements, reversible on/off motion and the tuning of active wavelengths by tuning the micromotor composition, which are difficult to obtain in chemically driven micromotors1. Hence, there is increasing interest in the synthesis and characterization of light-active materials as nano-/micromotors. For instance, TiO22,3, ZnO4, and AgCl5,6 have been used as photoactive materials under UV irradiation. However, there is growing interest in developing new nanomaterials that are capable of producing motion under visible light irradiation, such as Cu2O7, SiO2/Au/PEN8, and BiVO49, sulfur- and nitrogen-containing porous donor–acceptor polymers (SNPs)10 or C3N411. Ag3PO4 is a promising photocatalytic material due to its high separation of photogenerated electron/hole pairs facilitated by the absorption of visible light. This enables the degradation of organic pollutants and the production of oxygen from water splitting. Different strategies for improving Ag3PO4 photoactivity have been reported, such as its combination with other materials to form composites or heterostructures and in crystal facet engineering12. Despite its promising capabilities as a visible-light driven photocatalyst, it has been scarcely used for the development of micromotors. Ag3PO4 micromotors have been proposed as active colloids using UV light13 or chemical14 stimuli in their spherical form containing mixed facets and as visible light-driven micromotors in their cubic form15. The controllable synthesis of light-driven micromotors is still a relevant area of research in the field of improving micromotor capabilities. In recent years, different structures, such as microtubes11,16 and Janus spheres17,18,19, have been successfully implemented in micro-/nanorobots using different catalytic materials. However, even considering the critical role that facets have in the photocatalytic performance of a semiconductor, it remains unexplored in the micromotor field except for a very recent paper by Liu et al.20, which studied the effects of facets on Cu2O micromotor motion.
Many studies are conducted with interest in the application of micromotors in combatting bacterial contamination. The abilities to produce motion and carry potential antibacterial compounds are promising tools for the combination of mechanical eradication and biochemical interactions between micromotors and bacterial biofilms21,22,23,24,25.
The ability of bacteria to form biofilms is a dangerous kind of virulence that causes threats in the surgery field (risk of gram-positive methicillin-resistant Staphylococcus aureus (MRSA) presence) and in the food industry, such as the dairy and meat industries (risk of gram-negative Pseudomonas aeruginosa (P. aeruginosa) presence)26. The removal of biofilms by commonly used sanitary compounds is impossible to conduct in hard-to-reach places. By combining the mechanical eradication of biofilms by micromotors with the cell death of biofilm bacteria, a modern and sophisticated method is facilitated for application in biofouling clean-up processes in many industries27.
Therefore, in this work, the effects of the presence of different exposed facets on the motion of visible-light driven Ag3PO4 photocatalytic micromotors was evaluated. In addition, taking advantage of the antibacterial properties of Ag3PO4, their ability to eliminate the bacterial biofilms of P. aeruginosa and MRSA bacteria was observed in the absence of H2O2 and UV light.
Results and discussion
Preparation and characterization of Ag3PO4 photocatalytic micromotors
The effects of different crystal facets on the photocatalytic properties of Ag3PO4 have been studied in recent years to improve its performance in the photodegradation of pollutants28,29, water splitting12,30,31 and biofilm eradication32,33,34. Even if some reports in the literature were conducted with regard to the motion of Ag3PO4-based particles, only cubic and amorphous particles have been separately studied. However, the effects of different shapes and facets of different Ag3PO4 particles remain unexplored. Therefore, the main objective of this work was to explore the effects of crystal facet engineering on Ag3PO4-based micromotor motion and antibiofilm properties (Fig. 1A). To investigate the relationships between facets and motion, different shapes of Ag3PO4 micromotors were synthetized using a cheap and scalable precipitation method. Amorphous Ag3PO4 (am-Ag3PO4) containing mixed facets, cubic Ag3PO4 (c-Ag3PO4) with dominating {100} facets and tetrahedral Ag3PO4 (t-Ag3PO4) with dominating {111} facets were synthesized. Am-Ag3PO4 was synthetized by the direct precipitation of AgNO3 with NaH2PO4, while in the case of c-Ag3PO4, a silver/ammonia complex was formed before the addition of NaH2PO4. The motion mechanism for light-powered Ag3PO4-based nanocarriers is schematically illustrated in Fig. 1B. Visible light photons having energies equal to or higher than the Ag3PO4 bandgap are absorbed, promoting electrons (e−) from the valence band (VB) to the conduction band (CB) and leaving holes in the VB. The energy of the holes created by visible light is sufficiently high to oxidize water, producing oxygen. However, the energy of the electrons is lower than that of H+/H2; hence, without the addition of an electron scavenger, Ag3PO4 itself will decompose during water photooxidation, producing Ag on the surface and realizing the formation of Ag+ ions13,35. The asymmetric generation of chemical species on the surface of the Ag3PO4 micromotors is mainly responsible for their motion15. Since the photogenerated ions diffuse at different rates, a local electric field is generated due to the asymmetric charge distribution around the motor, moving the particles forward due to a self-diffusiophoretic mechanism9,36. Due to the high activity of this reaction, Ag3PO4 is able to display self-propulsion in fuel-free conditions in deionized water.
As seen in the micrographs shown in Fig. 2, the successful syntheses of am-Ag3PO4, c-Ag3PO4 and t-Ag3PO4 were accomplished. Additionally, the EDS mapping confirmed the presence of the constituent elements Ag, P and O, confirming the particle composition. However, a larger cluster of particles retaining the shape and exposed facets were also observed, as shown in Fig. S1.
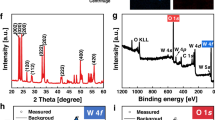
To confirm the dominating facets in the prepared samples, their XRD spectra were recorded and investigated. The X-ray diffraction patterns shown in Fig. 3A clearly indicate a full phase match with the body-centered cubic crystalline structure of Ag3PO4 (6.004 Å, BCC, JCPDS no. 06-0505). No additional peaks were found in the XRD signals, indicating well-crystallized pure-phase compounds for all the structures and the absence of impurities. The c-Ag3PO4 showed an intensity ratio of 0.35 between the peaks of the {110} and {200} planes, which is weaker than that in the am-Ag3PO4 (0.65) and t-Ag3PO4 (0.63) planes. This indicates the preferential exposure of {100} facets in c-Ag3PO4. In addition, the ratio between the {222} and {200} planes is higher in the case of t-Ag3PO4 (1.20) than in the cases of am-Ag3PO4 (1.01) and c-Ag3PO4 (0.43). These ratios indicate the preferential exposure of {111} facets in t-Ag3PO430,37. The UV–vis spectra (UV–VIS) of the different particles are presented in Fig. 3B. All samples showed an absorption edge at approximately 500 nm, confirming their visible light absorption.
Motion behavior of Ag3PO4 photocatalytic micromotors
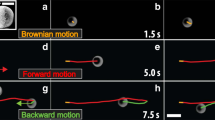
The motion of the different synthesized Ag3PO4 micromotors can be observed in Videos S1 to S3 for t-Ag3PO4, c-Ag3PO4 and am-Ag3PO4, respectively. The particles were tracked using Trackmate, and the resulting trajectories were extracted and plotted from the coordinate origin of each particle (Fig. 4A). Particle movement was tracked over 10 s under dark and illuminated conditions using a visible light microscope source, and the track length was dramatically increased from the dark to the illuminated conditions, confirming the visible light-powered motion mechanism of the particles. The Ag3PO4 micromotor trajectories were studied by performing mean-squared displacement (MSD) analysis under different dark and visible light irradiation conditions in deionized water. The MSD (µm2) for a given time interval (Δt) is defined as follows, where < > indicates an assembly of n particles:
A Groupings of the analyzed trajectories of Ag3PO4 micromotors over 10 s plotted from a single origin under dark (lightbulb OFF) and visible illumination (lightbulb ON). B MSD values of Ag3PO4 micromotors under dark conditions (squares) and visible light illumination (circles). Each row corresponds to a micromotor shape; from top to bottom: t-Ag3PO4, c-Ag3PO4 and a-Ag3PO4. C Diffusion coefficients calcuted from MSD of t-Ag3PO4 (blue), c-Ag3PO4 (red) and a-Ag3PO4 (black). D Tafel plots of t-Ag3PO4 (blue), c-Ag3PO4 (red) and a-Ag3PO4 (black).
In the case of pure Brownian motion, the MSD obeys the following relationship:
where D (µm2s−1) is the diffusion coefficient of the particles.
In the case of ballistic motion, the MSD obeys the following relationship:
The time interval or frame rate used (50 FPS) was set below the rotational diffusion (τR), where ballistic motion dominates over rotational motion. τR was calculated using the following equation:
where η is the viscosity (0.89 mPa), kB is the Boltzmann constant, T is the temperature (25 °C) and R is the particle radius. τR was estimated to be 13 s, and hence, the MSD was studied for t < 1 s (t < τR). Under these conditions, the motion mechanisms of the Ag3PO4-based micromotors containing different facets were studied through their MSD analyses. Under dark conditions, the MSD values of all three structures increased linearly over time, indicating Brownian motion, whereas under visible light irradiation, increased parabolically, indicating ballistic motion (Fig. 4B). From the MSD fitting, the diffusion coefficients of the micromotors were calculated and are plotted in Fig. 4C. The mixed potential of a semiconductor under dark and light conditions provides information about the corresponding catalytic process. As seen in Fig. 4D, the mixed potential values of the different Ag3PO4 micromotors were shifted toward more anodic potentials following the order of ΔEAg3PO4Light:dark = 73, 51, and 29 mV for t-Ag3PO4, c-Ag3PO4 and a-Ag3PO4, respectively. The positive shift in the mixed potential under irradiation indicates the tendency of the material to be reduced, which is associated with the photocorrosion of Ag3PO4 into Ag, which is related to micromotor motion and O2 generation. Interestingly, these differences in the mixed potentials were correlated with the diffusion coefficients (D) of the Ag3PO4 micromotors. Therefore, the trend found in the motion capability is in agreement with previous reports wherein {111} facets exhibited a higher photocatalytic activity than the {100} facets, confirming that we can tune the motion of Ag3PO4 micromotors using faceting nanoarchitectonics30,37. Figure 5 shows the strong fluorescence exhibited by the Ag3PO4-based micromotors under 488 nm excitation. By taking advantage of their inherent fluorescence, the motors can also be tracked by fluorescence microscopy.
Biofilm eradication using Ag3PO4 photocatalytic micromotors
Our group’s previous work studied the application of micromotors in targeting gram-positive and gram-negative biofilms. The motion of micromotors was induced by the presence of both 1 wt% H2O2 and UV light exposure20. Despite the excellent eradication effect, H2O2 at high concentrations and UV exposure are still invasive for nontargeted bacteria and other surrounding organisms. In this study of micromotors, motion is induced by visible light along without the need for H2O2. The am-Ag3PO4, c-Ag3PO4, and t-Ag3PO4 micromotors were tested on two different bacterial monospecies biofilms. The bacterial strains used for the biofilm eradication tests were gram-positive in the case of methicillin-resistant Staphylococcus aureus (MRSA) and gram-negative in the case of Pseudomonas aeruginosa (P. aeruginosa).
Viability assays were performed under three different conditions: control (indicated as C, bacteria under light exposure), in the presence of static micromotors (1 µg/mL) under dark conditions (S) and using the same amount of moving micromotors under light irradiation (M). As shown in Fig. 6A, the presence of micromotors dramatically affected biofilm viability, which was highly decreased in the presence of static micromotors (66%, 74% and 45% for am-Ag3PO4, c-Ag3PO4, and t-Ag3PO4, respectively) and decreased to a greater extent under moving conditions (17%, 57% and 15% for am-Ag3PO4, c-Ag3PO4, and t-Ag3PO4, respectively). In addition to biofilm viability, the influences of the micromotors on biofilm thickness were evaluated with positive results. The treatment of gram-negative Pseudomonas aeruginosa with all micromotor shapes resulted in significant decreases in biofilm thickness, from 77 µm in the control to 45, 38, and 18% with respect to the control values for am-Ag3PO4, c-Ag3PO4, and t-Ag3PO4, respectively (Fig. 6B). Additionally, the confocal microscope images of the biofilm shown in Fig. S4 indicate a sparser and weaker biofilm after treatment.
A control without the presence of particles and light irradiation was added. A Determination of the biofilm viability of P. aeruginosa after treatment with a-Ag3PO4, c-Ag3PO4, and t-Ag3PO4 micromotors. B Measurement of P. aeruginosa biofilm thickness after exposure to light-propelled micromotors. C (control): without the presence of motors or light, S (static): in the presence of micromotors and M (moving): in the presence of micromotors and light.
The treatment of the biofilm of methicillin-resistant S. aureus (MRSA) by micromotors was significantly more effective in comparison with that of P. aeruginosa biofilm. In this case, the viability assay, as shown in oltzmann constant, T is the tem Fig. 7A, showed that in the presence of static micromotors, lower viability was recorded in comparison to that of P. aeruginosa (1%, 32% and 18% for am-Ag3PO4, c-Ag3PO4, and t-Ag3PO4, respectively), which decreased to a greater extent under moving conditions (0.5%, 4% and 4% for am-Ag3PO4, c-Ag3PO4, and t-Ag3PO4, respectively). The thickness of the biofilm also decreased in comparison to that of the control, from 19 µm for the control to 9, 8 and 13 µm for am-Ag3PO4, c-Ag3PO4, and t-Ag3PO4, respectively. The biofilm again appeared sparser than the control (Fig. S5).
A control without the presence of particles and light irradiation was added. A Determination of the biofilm viability of MRSA after treatment with a-Ag3PO4, c-Ag3PO4, and t-Ag3PO4. B Measurement of MRSA biofilm thickness after exposure to light-propelled micromotors. C (control): without the presence of motors or light, S (static): in the presence of micromotors and M (moving): in the presence of micromotors and light.
Differences in the effects on MRSA and P. aeruginosa are caused by the arrangement and composition of the biofilm matrix and by the molecular composition of the bacterial gram-positive and gram-negative cell walls. Previous reports, such as those in the study performed by Choi et al., mentioned that the pathogen autoaggregation ability of S. aureus is 2 times higher than that of P. aeruginosa. The reason for this may be the significantly higher ability of P. aeruginosa to produce proteins, carbohydrates, and eDNA in its biofilms compared to that of MRSA, which could explain the different efficiencies of biofilm eradication observed in this work38.
Conclusions
In this work, we developed fuel-free Ag3PO4 micromotors powered by visible light irradiation. The Ag3PO4-based micromotors were synthetized with different exposed facets to study the corresponding effects on their motion. t-Ag3PO4 exhibited the highest motion capability, which is in accordance with its more active facets. Moreover, the Ag3PO4-based micromotors exhibited high inherent fluorescence, which can be advantageous for their tracking. Furthermore, the proposed Ag3PO4-based micromotors were employed for the biofilm eradication of gram-positive and gram-negative bacterial biofilms, demonstrating enhanced biofilm eradication under motion conditions compared with that under static conditions. These results show the potential of crystal facet engineering in the design of new micromotors for tuning their motion and also demonstrate the use of low-cost visible light-driven fuel-free photocatalytic-based micromotors as alternatives to other previously reported antibiofilm micromotors requiring the use of both UV light and H2O2.
Material and methods
Synthesis
Different Ag3PO4 shapes were synthetized by a simple and scalable precipitation method as follows. For t-Ag3PO4, 43 mg AgNO3 was added to a beaker containing 2 mL of ethanol to form a homogeneous solution. The solution was added dropwise to a reaction flask containing 10 mL of 0.1 M H3PO4 ethanol solution at 60 °C, forming a precipitate. In the case of c-Ag3PO4, the formation of the [Ag(NH3)2]+ complex was required prior to Ag3PO4. To achieve this, 100 mg AgNO3 was added to 10 mL deionized water, and 0.1 M ammonia solution was added dropwise. First, a dark precipitate of silver oxides appeared, which was further dissolved by adding an excess of NH3 to form the [Ag(NH3)2]+ complex. Then, 0.15 M Na2HPO4 solution was added dropwise to the [Ag(NH3)2]+ solution to form a precipitate. For a-Ag3PO4, 10 mL of 0.15 M Na2HPO4 was added dropwise to 30 mL of 0.15 M AgNO3 to obtain a precipitate. The obtained yellow precipitates were collected by centrifugation and washed with deionized water and ethanol four times. Finally, the obtained powders were dried in an oven at 60 °C for 12 h37,39.
Physicochemical characterization
A Tescan MIRA 3 XMU SEM equipped with an EDX detector (Oxford Instruments) was used for morphology characterization and elemental mapping. The crystallinity was studied by X-ray diffraction (XRD) using an X-ray diffractometer (Rigaku SmartLab 3 kW) with a Brag Brentano geometry and Cu Kα radiation. UV–Vis spectra were recorded using a Jasco V-750 UV–Vis spectrophotometer.
Electrochemical measurements
Prior to Tafel experiments, 5 μL of a Ag3PO4 suspension (1 mg mL–1) was dropcast onto a screen-printed electrode to produce a working electrode. An Ag/AgCl/0.1 M KCl electrode was used as a reference electrode, and a Pt wire was used as a counter electrode. Deionized water was used as an electrolyte to mimic the conditions of the movement experiments. Tafel plot measurements were carried out with an Autolab potentiostat (PGSTAT 204, Metrohm) with NOVA software 2.1 at a scan rate of 5 mV s–1. The irradiation source was a customized setup consisting of light-emitting diodes (LZ4-40B208, LedEngin Inc.) with a wavelength of 460 nm.
Movement characterization
For the movement characterization, a-Ag3PO4, c-Ag3PO4 and t-Ag3PO4 dispersions at concentrations of 0.05 mg were placed on glass slides that were then observed using 40x and 60x objectives (Nikon ECLIPSE TS2R inverted microscope). Videos were recorded using a Hamamatsu digital C13440-20CU camera at a frame rate of 50 FPS. The light flux (160 mW/cm2) was determined by measuring the light power passing through the sample position (Optical power meter S305C, Thorlabs, USA). The recorded videos were tracked using the Trackmate plugin for ImageJ40, and MSD values were calculated using the msdanalyzer41.
Biofilm eradication experiments
a-Ag3PO4, c-Ag3PO4 and t-Ag3PO4 were used for the elimination of monospecies bacterial biofilms. Gram-positive methicillin-resistant S. aureus (CCM 7110) and gram-negative Pseudomonas aeruginosa (CCM 3955) bacteria obtained from the Czech Collection of Microorganisms (CCM, Brno, Czech Republic) were tested for biofilm eradication in 96-well plates using the following protocol: Fresh bacterial culture was diluted in brain heart infusion broth (BHI) to an optical density of 0.1 (OD600), and 200 μL of bacterial inoculum was pipetted into 96-well U-shaped plates for 7 days of incubation at 37 °C. The BHI medium was changed regularly every day. After incubation, the biofilm plates were washed three times with phosphate-buffered saline (PBS). Micromotors were used at a concentration of 1 μg mL−1. The experiments were performed in four replicates, and error bars are expressed as the standard deviation. Plates with biofilm and micromotor samples were irradiated using an LED lamp (Fig. S3 for emission spectra) to enhance biofilm eradication ability, and the controls were prepared at the same time. Measurements of biofilm viability were performed at 60 min, and then the biofilms were washed three times with PBS. Biofilm viability was determined by Alamar Blue staining (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The fluorescence of each well was evaluated (560/590 nm, excitation/emission). 3D biofilm images and thicknesses were collected using confocal scanning microscopy as described in our previous study20.
References
Yuan, K., Bujalance-Fernández, J., Jurado-Sánchez, B. & Escarpa, A. Light-driven nanomotors and micromotors: envisioning new analytical possibilities for bio-sensing. Microchimica Acta. 187, 1–16 (2020).
Maric, T., Nasir, M. Z. M., Webster, R. D. & Pumera, M. Tailoring Metal/TiO2 Interface to Influence Motion of Light-Activated Janus Micromotors. Adv. Funct. Mater. 30, 1908614 (2020).
Dong, R., Zhang, Q., Gao, W., Pei, A. & Ren, B. Highly efficient light-driven TiO2–Au Janus micromotors. ACS Nano. 10, 839–844 (2015).
Pourrahimi, A. M., Villa, K., Sofer, Z. & Pumera, M. Light-driven sandwich ZnO/TiO2/Pt Janus micromotors: Schottky barrier suppression by addition of TiO2 atomic interface layers into ZnO/Pt micromachines leading to enhanced fuel-free propulsion. Small Methods 3, 1900258 (2019).
Zhou, C., Zhang, H. P., Tang, J. & Wang, W. Photochemically powered AgCl Janus micromotors as a model system to understand ionic self-diffusiophoresis. Langmuir 34, 3289–3295 (2018).
Wang, X., Baraban, L., Nguyen, A., Ge, J., Misko, V. R. & Tempere, et al. High-motility visible light-driven Ag/AgCl Janus micromotors. Small 14, 1803613 (2018).
Wang, Q. et al. Glucose-fueled micromotors with highly efficient visible-light photocatalytic propulsion. ACS Appl. Mater. Interfaces 11, 6201–6207 (2019).
Sun, Y. et al. Visible light-driven micromotor with incident-angle-controlled motion and dynamic collective behavior. Langmuir 37, 180–187 (2021).
Villa, K. et al. Visible-light-driven single-component BiVO4 micromotors with the autonomous ability for capturing microorganisms. ACS. Nano. 13, 8135–8145 (2019).
Kochergin, Y. S. et al. Multifunctional visible-light powered micromotors based on semiconducting sulfur- and nitrogen-containing donor–acceptor polymer. Adv. Funct. Mater. 30, 2002701 (2020).
Villa, K., Palenzuela, C. L. M., Sofer, Z., Matějková, S. & Pumera, M. Metal-free visible-light photoactivated C3N4 bubble-propelled tubular micromotors with inherent fluorescence and on/off capabilities. ACS. Nano. 12, 12482–12491 (2018).
Hasija, V. et al. A strategy to develop efficient Ag3PO4-based photocatalytic materials toward water splitting: Perspectives and challenges. Chem. Cat. Chem. 13, 2965–2987 (2021).
Altemose, A., Harris, A. J. & Sen, A. Autonomous formation and annealing of colloidal crystals induced by light-powered oscillations of active particles. Chem.Sys. Chem. 2, e1900021 (2020).
Altemose, A. et al. Chemically controlled spatiotemporal oscillations of colloidal assemblies. Angew. Chem. Int. Ed. 56, 7817–7821 (2017).
Chen, C. et al. Light-steered isotropic semiconductor micromotors. Adv. Mater. 29, 1603374 (2017).
Asunción-Nadal, V., de la, Pacheco, M., Jurado-Sánchez, B. & Escarpa, A. Chalcogenides-based tubular micromotors in fluorescent assays. Anal. Chem. 92, 9188–9193 (2020).
María-Hormigos, R. et al. Oscillatory light-emitting biopolymer based Janus microswimmers. Adv. Mater. Interfaces 7, 1902094 (2020).
Pacheco, M., Jurado-Sánchez, B. & Escarpa, A. Visible-light-driven janus microvehicles in biological media. Angew. Chem. 131, 18185–18192 (2019).
Urso, M., Ussia, M. & Pumera, M. Breaking polymer chains with self-propelled light-controlled navigable hematite microrobots. Adv. Funct. Mater. 31, 2101510 (2021).
Liu, W. et al. Visible-light-driven cuprous oxide nanomotors with surface-heterojunction-induced propulsion. Nanoscale Horiz. 6, 238–244 (2021).
Ussia, M. et al. Active light-powered antibiofilm ZnO micromotors with chemically programmable properties. Adv. Funct. Mater. 31, 2101178 (2021).
Yuan, K., Jurado-Sánchez, B. & Escarpa, A. Dual-propelled lanbiotic based janus micromotors for selective inactivation of bacterial biofilms. Angew. Chem. Int. Ed. 60, 4915–4924 (2021).
Ge, Y. et al. Dual-fuel-driven bactericidal micromotor. Nano-Micro Lett. 8, 157–164 (2015).
Delezuk, J. A. M., Ramírez-Herrera, D. E., Ávila, B. E.-Fde & Wang, J. Chitosan-based water-propelled micromotors with strong antibacterial activity. Nanoscale 9, 2195–2200 (2017).
Yuan, K., Jiang, Z., Jurado-Sánchez, B. & Escarpa, A. Nano/Micromotors for Diagnosis and Therapy of Cancer and Infectious Diseases. Chem. – A Eur. J. 26, 2309–2326 (2020).
Cao, Y. et al. Non-antibiotic antimicrobial agents to combat biofilm-forming bacteria. J. Glob. Antimicrobial Resistance 21, 445–451 (2020).
Shi, Z. et al. Rationally designed magnetic poly(catechol-hexanediamine) particles for bacteria removal and on-demand biofilm eradication. Colloids Surf. B: Biointerfaces 186, 110728 (2020).
Ge, M., Zhu, N., Zhao, Y., Li, J. & Liu, L. Sunlight-assisted degradation of dye pollutants in Ag3PO4 Suspension. Ind. Eng. Chem. Res. 51, 5167–5173 (2012).
Teng, F., Liu, Z., Zhang, A. & Li, M. Photocatalytic performances of Ag3PO4 polypods for degradation of dye pollutant under natural indoor weak light irradiation. Environ. Sci. Technol. 49, 9489–9494 (2015).
Martin, D. J., Umezawa, N., Chen, X., Ye, J. & Tang, J. Facet engineered Ag3PO4 for efficient water photooxidation. Energy Environ. Sci. 6, 3380–3386 (2013).
He, G. et al. Facile controlled synthesis of Ag3PO4 with various morphologies for enhanced photocatalytic oxygen evolution from water splitting. RSC Adv. 9, 18222–18231 (2019).
Tran, H. A. & Tran, P. A. Immobilization-enhanced eradication of bacterial biofilms and in situ antimicrobial coating of implant material surface; an in vitro Study. Int. J. Nanomed. 14, 9351–9360 (2019).
Steckiewicz, K. P. et al. Shape-Depended Biological Properties of Ag3PO4 Microparticles: Evaluation of Antimicrobial Properties and Cytotoxicity in Vitro Model - Safety Assessment of Potential Clinical Usage. Oxidative Med. Cellular Longevity 2019, 6740325 (2019).
Blanchette, V., Belosinschi, D., Lai, T. T., Cloutier, L. & Barnabé, S. New Antibacterial Paper Made of Silver Phosphate Cellulose Fibers: A Preliminary Study on the Elimination of Staphylococcus aureus Involved in Diabetic Foot Ulceration. BioMed Res. Int. 2020, 1304016 (2020).
Yi, Z. et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 9, 559–564 (2010).
Hong, Y., Diaz, M., Córdova-Figueroa, U. M. & Sen, A. Light-Driven Titanium-Dioxide-Based Reversible Microfireworks and Micromotor/Micropump Systems. Adv. Funct. Mater. 20, 1568–1576 (2010).
He, S. et al. Femtosecond time-resolved diffuse reflectance study on facet engineered charge‐carrier dynamics in Ag3PO4 for antibiotics photodegradation. Appl. Catal. B: Environ. 281, 119479 (2021).
Choi, N.-Y., Bae, Y.-M. & Lee, S.-Y. Cell surface properties and biofilm formation of pathogenic bacteria. Food Sci. Biotechnol. 24, 2257–2264 (2015).
Bi, Y., Ouyang, S., Umezawa, N., Cao, J. & Ye, J. Facet effect of Single-Crystalline Ag3PO4 Sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 133, 6490–6492 (2011).
Tinevez, J. Y. et al. TrackMate: An open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Tarantino, N. et al. TNF and IL-1 exhibit distinct ubiquitin requirements for inducing NEMO–IKK supramolecular structures. J. Cell Biol. 204, 231–245 (2014).
Acknowledgements
This work was supported by the project Advanced Functional Nanorobots (reg. No. CZ.02.1.01/0.0/0.0/15_003/0000444 financed by the EFRR). M.P. was also supported by Ministry of Education, Youth and Sports (Czech Republic) grant LL2002 under ERC CZ program. K.D. and M.K. acknowledge financial support from ERDF “Multidisciplinary research to increase application potential of nanomaterials in agricultural practice” (No. CZ.02.1.01/0.0/0.0/16_025/0007314).
Author information
Authors and Affiliations
Contributions
D.R. designed the experiments, synthesized the Ag3PO4 micromotors, and performed SEM-EDX, XRD, UV–Vis, electrochemical measurements, and motion analysis. M.K. participated in biofilm preparation, treatment optimization, and the determination of biofilm viability. K.D. prepared and treated the biofilms for confocal microscopy and biofilm thickness measurements, supervised the design of the microbiological analyses and contributed to writing of the manuscript. M.P. conceptualized the research and supervised the micromotor-based work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rojas, D., Kuthanova, M., Dolezelikova, K. et al. Facet nanoarchitectonics of visible-light driven Ag3PO4 photocatalytic micromotors: Tuning motion for biofilm eradication. NPG Asia Mater 14, 63 (2022). https://doi.org/10.1038/s41427-022-00409-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-022-00409-0