Abstract
A high-frequency magnetic field (MF) generates an electric current by charging conductors that enable the induction of various biological processes, including changes in cell fate and programming. In this study, we show that electromagnetized carbon porous nanocookies (NCs) under MF treatment facilitate magnetoelectric conversion for growth factor release and cell stimulation to induce neuron cell differentiation and proliferation in vitro and in vivo. Integrating four-dimensional printing technology, the NCs are exposed on the surface, which enhances the cell adhesion and allows direct manipulation of electromagnetic stimulation of the cells. Remarkably, large amounts of growth factor encapsulated in NC@conduit resulted in excellent permeability and on-demand release, improving the in vivo layers of myelin sheaths and directing the axon orientation at 1 month postimplantation. This study offers proof of principle for MF-guided in vivo neuron regeneration as a potentially viable tissue regeneration approach for neuronal diseases.
Similar content being viewed by others

Introduction
Four-dimensional (4D) printed soft materials from the molecular design of a three-dimensional (3D) printed object capable of transforming intrinsic properties over time in response to environmental stimuli, such as voltage, temperature, and chemical reaction, are of considerable interest in soft microrobotics, computational folding, and memory materials1,2,3,4,5. Printed complex geometries offering personal biomedical engineering applications are required in new drug delivery formulations6, tumor therapy7,8, living devices9, and actuators10,11. For example, 3D printed tablets or oral compartmental dosage forms exhibit a controllable-by-design delivery system to program drug release patterns12. In the regeneration of volumetric tissue, 3D cell printing scaffolds release cell or growth factors to stimulate blood vessel reconstruction and manipulate stem cell differentiation13. However, only a few 3D printing techniques can give a uniquely controllable surface roughness and on-demand electric current in a biomicroenvironment to induce various biological processes. Through modifying this technology, a versatile structure can be potentially optimized with the material design in one compact system to facilitate personalized medicine.
Even with the current breakthroughs in 3D printing, the challenges of printed tissue-regenerated materials still exist in on-demand biocues and electrical stimulus for programming neuron-related cells, which is a key factor to promote neurite sprouting and Schwann cell (SC) migration across the injury site14,15, especially for peripheral nerve injury (PNI)16,17. In addition, the electric current enables the induction of different biological processes, including changes in cell fate and programming18. However, deep nerve stimulus via electric current usually requires invasive implantation of an electrode in the tissue19. To address this issue, by combination with a nanoconductor, a high-frequency magnetic field (MF) can remotely induce electric currents (eddy currents) by charging objects (e.g., gold and graphene-based materials). Such physical energy has also been capable of controlling drug release or promoting osteogenesis and neurogenesis for cell proliferation20,21. Regarding neuron stimuli, Yoo and coworkers demonstrated that electromagnetized gold nanoparticles could facilitate the lineage reprogramming of dopamine neurons22. Nevertheless, these nanoconductors have to contact the cell directly, which is difficult to achieve by traditional hydrogel modeling since the particles are embedded in the gel rather than exposed on the surface. In contrast, 3D printing by light curing can expose the nanoconductors on the surface of the matrix and be integrated with an MF to stimulate cells due to the exquisite curing process, which inherently creates a broad strategy to affect tissue regeneration.
To amplify the functions of 3D printing, a simple approach is to integrate nanoconductors into printed soft matter. Graphene exhibiting biocompatibility and conductivity has been utilized in drug delivery23, stem cell differentiation24, sensors25,26, and tissue engineering27. By incorporating this material, 3D printing of graphene elastomers with excellent electrical, flexible, and neurogenic properties can expose graphene on the surface, and support of neurogenic differentiation28. Furthermore, Hsu et al. used 3D printed polyurethane (PU)-based hydrogel scaffolds with bioactive ingredients, which induced chondrogenic differentiation of mesenchymal stem cells for cartilage regeneration29. A commercial product, Opsite®, that was applied as a transparent adhesive PU-based dressing exhibited oxygen permeability and accelerated dermal repair30. Such PU-based scaffolds are beneficial to injured nerve tissue with long-term supports, including rigidity, topographic cues, and nutrient permeability31,32,33,34. These developments of 3D printed biocompatible elastomers offer potential opportunities for tissue engineering.
Here, we developed a 3D printing method to prepare a stretchable and biocompatible conduit with a nanocomposite composed of reduced graphene oxide (GO) nanosheets, nanocookies (NCs), for peripheral nerve regeneration. NCs with mesoporous silica exposed on the surface of the conduits provide magnetoelectric conversion, protein adsorption, and cell stimulation. The internal microchannels inside were simply and simultaneously prepared by an additive manufacturing process, in contrast to earlier studies34. On-demand neuron growth factor (NGF) release and cell stimulus were achieved in vitro under MF treatment to effectively induce cell proliferation and differentiation, and the implants promoted axon outgrowth after a peripheral nerve was transected 10 mm from the bifurcation point of the sciatic nerve of Sprague-Dawley (SD) rats.
Materials and methods
Synthesis of GO nanosheets and NCs
GO was synthesized via a modified Hummers method35. First, graphite powder (3.0 g) was mixed with concentrated H2SO4 (69 ml) and NaNO3 (1.5 g). After 5 min, the mixture was cooled in an ice bath to 0 °C. Then, KMnO4 (9.0 g) was added slowly because the reaction temperature had to be kept <20 °C. Then, the mixture was stirred and heated at 35 °C. After 12 h, 30% H2O2 was added to the mixture until the solution turned bright yellow. The mixture was washed by adding 30% HCl (200 ml) and centrifuged at 6000 r.p.m. for 10 min two times. The precipitates were collected and washed with deionized water until the pH of the washings became neutral. Finally, after drying, GO was collected. The synthesis of NCs was previously described36. Briefly, 0.1 g of cetyl trimethyl ammonium bromide (CTAB) and 0.4 mg GO were placed in 30 ml of deionized water, and the mixture was stirred at 60 °C for 30 min. Then, 14.4 ml of octane, 2.45 ml of styrene, 0.022 g of L-lysine, and 1.071 ml of TEOS were added into the mixture. After the mixture was mixed, 0.0371 g of AIBA was immediately added, and the solution was stirred at 60 °C for 3 h. Next, the heating stopped, and the mixture was cooled naturally to room temperature (~12 h). The mixture was washed with deionized water three times and then centrifuged at 6000 r.p.m. for 10 min. The gray powder was collected by drying under vacuum at 60 °C. Finally, the gray powder was heated at 450 °C for 3 h under N2 gas and washed with deionized water three times to remove impurities. The material was finally dried to a powder and termed NCs.
Preparation of 3D printed conduit
A digital light processing (DLP) 3D printer was purchased from MiiCraft (MiiCraft plus, Taiwan), which was controlled by a computer via an RJ-45 cable. The maximum build area was 43 mm × 27 m × 180 m, and the minimum thickness of the product was 5 µm, which was adjustable up to 200 µm. An LED projector with a wavelength of 405 nm at a power of 12.5 mW was equipped in the printer. 3D printed resin was prepared by some types of light-curable molecules, including 17.8 g of 4-hydroxybutyl acrylate (4-HBA), 2 g of urethane-polyethylene glycol-polypropylene glycol (PU-EO-PO) monomer, and 0.2 g of Irgacure 819 as the initiator, which were well mixed and protected from light at room temperature for 3 h. Resins with 0.1% NCs were prepared by mixing 17.6 g of 4-HBA, 2 g of PU-EO-PO monomer, 0.2 g of Irgacure 819, and 0.02 g of NCs; resins containing 1% NCs were prepared by mixing 17.6 g of 4-HBA, 2 g of 230TH, 0.2 g of Irgacure 819, and 0.2 g of NCs. The resin underwent ultrasonication to avoid bubbles and precipitation. The 3D objects were drawn by 3D computer-aided design (CAD), and were then imported to slicer software (MiiUtility3.2) and uploaded to the 3D printer. The printing thickness per layer was 100 µm, and the curing time was 10 s per layer. After filling the printing pool with resin, the stage sank into the resin 100 µm from the bottom of the pool. The structure was polymerized and formed layer by layer on the stage. The 3D customized nerve guides were finally cured with further irradiation (postcuring under 18 W UVA lamp irradiation) at room temperature followed by washing with deionized water twice, and sterilization via an autoclave at a temperature of 121 °C at 15 psi for further in vitro and in vivo experiments.
Characterizations of NCs and the 3D printed conduit
The structure of the NCs was revealed by transmission electron microscopy (TEM, JEM-2100, Japan). The NCs were well dispersed in deionized water and dried on carbon film-supported copper grids under vacuum at room temperature for specimen preparation. Raman spectroscopy was used to determine the nonpolar bonds of graphite, GO, and NCs. All powders were dried on a silicon wafer and measured with a UniNanoTech system (UniRAM, South Korea) at 1.58 eV by excitation with a 514 nm laser. Dynamic light scattering (DLS) analysis by using a particle sizer (Nano-ZS, Malvern, UK) determined the average size, and size distribution of GO and NCs diluted with deionized water. N2 adsorption–desorption isotherms were measured by using an Autosorb 1 MP system (Quantachrome Instruments, Florida, USA). The Barrett–Joyner–Halenda (BJH) and Brunauer–Emmett–Teller (BET) methods were used to calculate the pore size and surface area of the samples, respectively. Graphite, GO, and NCs were dried at 70 °C under vacuum for 24 h, and degassed at 200 °C for 2 h before analysis. Field-dependent magnetization curves were evaluated by a superconducting quantum interference device (SQUID, Quantum Design MPMS-XL7, USA) from −40,000 to 40,000 Oe at 6 K and 300 K. The field-sensitive temperature dependence of magnetization was evaluated from 5 K to 300 K at 50 Oe. Atomic configurations and the surface elemental composition were examined by X-ray photoelectron spectroscopy (XPS, PHI Quantera II). The morphologies of the 3D printed conduit were determined by field emission scanning electron microscopy (FE-SEM, JSM-7000F, Japan). The samples were anchored to SEM specimen mounts using double-sided carbon tape and sputter deposited with platinum at 10 mA for 120 s. We followed ASTM-D638-14 to print the dog-bone shape for tensile testing (4468, Instron, USA) with a 1 kN load cell under a constant strain rate of 10 mm per min. Prior to all tests, the load was reset, and the load cell was calibrated. The printed samples were then postured for 0, 1, and 4 h to evaluate the stress and strain diagram (n = 6). The slope of the linear region from the stress–strain curve was calculated as Young’s modulus. The ultimate tensile strength was taken as the maximum stress reached before failure.
Cell culture and in vitro biocompatibility of 3D printed matrix
HIG-82 cells were cultured in Ham’s F12 medium containing 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin. Neuro-2a (N2a) cells were cultured in EMEM containing 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin. SCs were cultured in DMEM containing 0.094 g l−1 D-valine, 10% (v/v) FBS, 1% (v/v) N2 supplement, 20 μg ml−1 (wt/v) bovine pituitary extract, 5 μM forskolin, and 1% (v/v) penicillin/streptomycin. PC12 cells were cultured in RPMI-1640 with 10% (v/v) HS, 5% (v/v) FBS, and 1% (v/v) penicillin–streptomycin. The cells were cultured in complete medium at 37 °C in a humidified atmosphere of 5% carbon dioxide in air. The medium was changed every other day. The cytotoxicity followed the tests for in vitro cytotoxicity (ISO 10993-5). Briefly, we prepared samples in 1 × 1 × 0.1 cm3 by 3D printing and then washed them with deionized water, as well as ethanol three times. The samples were sterilized via an autoclave at a temperature of 121 °C at 15 psi for 30 min and extracted in culture medium with serum following ISO 10993-12. After seeding cells in 96-well plates (10,000 cells per well) for 1 day, we added the extraction into each well for another 1-day incubation. Finally, we used the MTT agent to detect toxicity at wavelengths of 570 nm and 650 nm as a reference. Cell viability was calculated by comparison with untreated cells and calculated according to the following: cell viability (%) = absorbance of experimental group/absorbance of control group. For the external MF test, we applied an MF above the cell culture dish 1 cm away at a power of 3.2 kW and frequency of 1 MHz for 15 min before using the MTT agent.
For observing cells on the 3D printed matrix by electron microscopy, cells that grew on the 3D printed matrix were further fixed with 2.5% glutaraldehyde for 30 min and then 2% osmium tetroxide (OsO4) in PBS. Cells were then washed three times with PBS for 10 min each followed by dehydration through a graded series of ethanol sequentially at 10, 20, 30, 50, and 70% (30 min for each step), and immersed in 100% acetone for 30 min each.
Flow cytometry and the effect of NCs on PC12 cells
QDs were adopted to label NCs for tracking purposes in in vitro experiments. The QDs were encapsulated in NCs by first mixing 2.5 mg of QDs and 10 mg of NCs dissolved in chloroform solution, stirring for 24 h. Then, the samples, NCs/QDs, were dried under vacuum at 60 °C for 1 h. To remove free QDs, the samples were washed with deionized water three times and collected by centrifugation at 6000 r.p.m. for 8 min. Afterward, 500,000 PC12 cells were cultured in six-well plates with 1 ml of medium for 24 h, and then incubated with NCs/QDs for 0.5, 1, and 4 h. Next, the cells were washed with PBS and soaked in trypsin-EDTA. After harvesting the cells with the culture medium, the cells were centrifuged at 500 r.p.m. for 5 min. Finally, the cells were rinsed with PBS and collected for flow cytometry analysis. In addition, 10,000 PC12 cells were seeded and grown on glass coverslips in wells at 37 °C for 24 h in the incubator, and then treated with NCs/QDs for 4 h. After incubation, cells were stimulated by MF (power cube 32/900, President Honor Industries, Taiwan) at a power of 3.2 kW and frequency of 1 MHz for 15 min. The cells were then continuously incubated for 3 days. The medium was removed, and the cells were washed twice with PBS for the dye procedure. We used 3.7% formaldehyde (PBS solution) to fix the cells for 20 min at 37 °C, and then washed the wells twice with PBS. Next, we permeabilized the cells by immersion in 0.1% Triton X-100 (diluted in PBS solution) for 20 min at 37 °C, and then washed the wells twice with PBS. Finally, we stained the nucleus and phalloidin by using DAPI (1 µg ml−1) and F-actin (1 unit ml−1) for 30 min, respectively. The images were obtained on a Zeiss LSM 780 CLSM equipped with an argon-ion laser.
Cell behaviors with physical cues and PC12 cell differentiation
3D printed conduits with and without microchannels were washed with both deionized water and ethanol three times. Then, the conduits were sterilized via an autoclave at a temperature of 121 °C at 15 psi for 30 min. Sterilized 3D printed planes were placed in six-well plates. Next, 10,000 HIG-82 cells with culture medium were seeded and cultivated on the 3D printed matrix for 1–4 days at 37 °C, and the cultures were then imaged, where DAPI stained the nucleus and phalloidin stained F-actin. The images were obtained on a Zeiss LSM 780 CLSM equipped with an argon-ion laser. The binary two-dimensional (2D) images were then processed by Nikon NIS-elements software, providing a basis for the printed physical cue orientation at 0°. The orientation was calculated by the angle at Feret’s maximum diameter. The resultant power spectra were then generated by plotting a Gaussian distribution fitting along a circumferential profile. For PC12 cell differentiation, 10,000 PC12 cells were seeded and grown on glass coverslips in the wells. The medium was changed to RPMI-1640 with 2% (v/v) HS, 1% (v/v) FBS, 1% (v/v) penicillin–streptomycin, and 100 ng ml−1 NGF in the control group for 5 days at 37 °C. Sterilized 3D printed planes were immersed in 100 ng ml−1 NGF for 2 days and then placed in six-well plates. Next, 10,000 PC12 cells were seeded and grown on the 3D printed planes with RPMI-1640 with 2% (v/v) HS, 1% (v/v) FBS, and 1% (v/v) penicillin–streptomycin at 37 °C for 1 day. Then, cells were stimulated by MF treatment above the cell culture dish 1 cm away at a power of 3.2 kW and frequency of 1 MHz for 15 min. The cells were then continuously incubated for 4 days. The medium was finally removed, and the cells were washed twice with PBS for the dye procedure. We used 3.7% formaldehyde (PBS solution) to fix the cells for 20 min at 37 °C, and then washed the wells twice with PBS. Next, we permeabilized the cells by immersion in 0.1% Triton X-100 (PBS solution) for 20 min at 37 °C and then washed the well twice with PBS. Finally, we stained the nucleus and phalloidin by using DAPI (1 µg ml−1) and F-actin (1 unit ml−1) for 30 min, respectively. The images were obtained on a Zeiss LSM 780 CLSM.
Animal and surgical procedure
All SD male rats aged 3 weeks were purchased from BioLASCO Taiwan Co., Ltd. All rats were maintained under conditions at 22 ± 2 °C on a 12 h dark day cycle with access to water and food. All experiments were approved by the guidelines of the Institution of Animal Care and Use Committee of National Tsing Hua University (NTHU). All rats were randomly selected to undergo repair with the left limb by a 3D printed conduit. Rats were fixed on the table, and the sciatic nerve was exposed by as small an incision as possible at the posterior of the left hind near the bifurcation point to allow both observation and the 3D printed conduit to be subcutaneously placed. In the autograft group, the sciatic nerve was cut 10 mm (gap) and inversely sutured with 9-0 sutures under a microscope, four times on each side. For the conduit group, muscle and skin incisions were closed by layers, and the animals could freely eat food and water after the surgery.
Functional assessment of walking track analysis
All paws of rats were recorded by a digital single-lens reflex camera postsurgery for 1 month. The feet of the injured limbs were measured with the following parameters: (1) toe spread (TS), the distance between the first and the fifth toes; (2) intermediate toe spread (ITS), the distance between the second and the fourth toes; and (3) Print length (PL), the distance from the heel to the third toe. The sciatic function index (SFI) was calculated (N: the contralateral hind limb; E: the experimental hind limb; a value of −100 implies total impairment): SFI = −38.3 (EPL − NPL)/NPL + 109.5 (ETS − NTS)/NTS + 13.3 (EITS − NITS)/NITS − 8.8.
Relative gastrocnemius muscle weight
All rats were sacrificed via inhalation of carbon dioxide postsurgery for 1 month. The regenerated nerve and adjacent nerve tissue were dissected in the container with 4% PFA (4 wt%) in 0.1 M PBS for 1 day. The gastrocnemius muscle of both hind limbs was also excised and weighed to analyze relative gastrocnemius muscle weight, and finally immersed in 4% PFA (4 wt%) in 0.1 M PBS for 3 days.
Immunohistochemistry
The harvested conduit with regenerative nerves was sequentially immersed in 10% (wt%) sucrose for 30 min, 15% (wt%) sucrose for 30 min, and 30% (wt%) sucrose for 30 min for dehydration. Then, the tissues were embedded in OCT, and horizontal and longitudinal cryostat sections (10 μm in thickness) were sliced, and stained for immunohistochemical analysis. In brief, the frozen sections were immersed in ethanol at −20 °C for 10 min and washed with PBS at room temperature three times for 5 min each. The sections were stained with anti-beta III-tubulin antibody [TU-20] (1:200, rabbit IgG1) for regenerated axons overnight at 4 °C, followed by goat anti-mouse IgG (H+L) antibody (FITC) for 2 h at room temperature. After washing three times with PBS, the sections were stained with mounting medium containing DAPI. Finally, all stained sections were visualized by a ZEISS LSM 780 microscope. The binary 2D images were then processed by Nikon NIS-elements software, providing a basis for the printed physical cue orientation at 0°. The orientation was calculated by the angle at Feret’s maximum diameter. The resultant power spectra were then generated by plotting a Gaussian distribution fitting along a circumferential profile.
Stereographic analysis of sciatic nerve regeneration
The harvested regenerative nerve (at the middle between each endpoint) was fixed with 3% glutaraldehyde at 4 °C and then postfixed with 1% OsO4. The specimens were serially dehydrated with ethanol and embedded in Araldite 502. The specimens were cut into semithin sections followed by staining with 1% toluidine blue. Ultrathin sections (60 nm) were lifted onto formvar-coated grids, poststained with lead citrate and uranyl acetate, and subsequently imaged using electron microscopy.
Results and discussion
Fabrication and characterization of NC@C
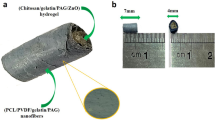
A composite conduit (NC@conduit, NC@C) integrating features of mesoporous carbon sheets and protein-permeable elastomers to promote peripheral nerve regeneration under magnetoelectric stimulation was developed in this study. As shown in Fig. 1a, a bioink composed of mesoporous carbon layers on a GO sheet (sandwich NCs) and 4-HBA/PU-EO-PO monomer was placed in a 3D printer resin bath. Then, UV light possessing a channel pattern in a ring was applied to cure the bioink to form a flexible composite NC@C with internal channels through DLP 3D printing. As shown in Fig. 1b, 3D printed NC@C loaded with a large payload of NGF was implanted to implement the spatial distribution of NGF for the promotion of axon outgrowth after a peripheral nerve was transected 10 mm from the bifurcation point of the sciatic nerve of SD rats. Upon receiving MF irradiation, the NFG encapsulated in NC@C showed excellent permeability and on-demand release to induce the differentiation and proliferation of nerve cells in vivo, while simultaneously supplying electromagnetic stimulation to cells. Via UV pattern design, conduits with various channels can be prepared and loaded with drugs or proteins in the matrix (Supplementary Fig. S1). After embedding NCs, the printed conduit and composite maintained good elastic and stretchable properties (Fig. 1c, d). A particular success of this strategy is the controllable roughness of the surface on a conduit by exposing NCs to 3D printing, which not only increases the roughness for cell adhesion, but also manifests as a direct physical stimulus to cells (Fig. 1e). Furthermore, through exquisite fabrication, the microchannels of NC conduits effectively promote the cell alignment (Fig. 1f).
a Composite bioink composed of 4-HBA, PU-EO-PO monomer, initiator, and NCs made through 3D printing for NC@C fabrication. b NC@C under MF treatment facilitated magnetoelectric conversion for growth factor release and cell stimulation to induce neuron cell differentiation. c Image of a 3D printed NC@C with elastic properties. d The elastic properties of NC@C showed stretchable properties. e SEM image of cells adhered on NC@C. The roughness of the exposed NCs on the surface improved cell attachment. f CLSM images of cells proliferating on NC@C with microchannels. DAPI-stained nuclei (red) and phalloidin-stained F-actin (green).
Synthesis and characterization of NCs
The robust composite NCs can be fabricated by employing GO as the substrate of the structure, as schematically illustrated in Fig. 2a. To prepare GO, the modified Hummers method was used. Similar to our previous work, a cationic surfactant (CTAB) was adsorbed onto the surface of synthesized GO via electrostatic interactions to form the micelle template. Under weakly basic conditions, the silica pore was constructed via the hydrolysis of tetraethyl orthosilicate and polymerization of styrene on the packed CTAB micelles on GO. Then, the particles were carbonized at high temperature under nitrogen to observe the NCs. TEM revealed that the mesoporous constructs were deposited on GO (Fig. 2b). With CTAB as the pore template, the pore size ranged from 3 to 6 nm in diameter, with an approximately random arrangement on the GO (Supplementary Fig. S2). Moreover, the chemical composition of graphite, GO, and NCs was estimated by XPS, and the analysis of the XPS patterns is displayed in Supplementary Fig. S3. A new, broad peak observed for GO at binding energies of 532 eV was attributed to O1s (ref. 37) and NCs at 154 and 174 eV for the Si2p and Si2s components, respectively (Supplementary Fig. S3a). Furthermore, to provide insight regarding the surface-decorated functional groups, deconvoluted XPS spectra of C1s O1s and Si2p are shown in Supplementary Fig. S3b–d in the Supporting Information. In addition to displaying the peaks of GO, the NCs exhibited the formation of C–Si and C–O–Si bonds in the structures. These results confirmed the successful exfoliation from graphite to GO by the Hummers method, and the attachment of carbon-containing silica onto the surface of the NCs. Furthermore, Raman spectroscopy was applied to evaluate the structure of graphite, GO, and NCs (Fig. 2c), using an excitation wavelength of 532 nm. All samples were deposited in powder form on silicon wafers without using any solvent. The peak of the G band is ~1614 cm−1 (in-phase sp2 bond vibration), and the D band is ~1360 cm−1 (disorder band of the graphene edge functional group). Two characteristic peaks were observed in all products. The relative peak ratio of G/D in graphene is higher than that in GO and NCs, which is probably caused by the low proportion of sp3 carbon centers (high content of sp2-hybridized C–C bonds)38.
a Schematic illustration of NC fabrication via the Hummers method and calcination. b TEM images of NCs. c Raman spectra of graphite, GO, and NCs. d Size distributions of graphite and NCs as determined by DLS. e N2 adsorption–desorption isotherms of NCs. f Field-dependent magnetization curves of NCs as determined by SQUID at 6 K and 300 K in the range of −40 kOe to 40 kOe. g NGF release of NCs with and without MF treatment for 15 min. h Flow cytometry analysis of the NCs in PC12 cells after incubation for 0.5, 1, and 2 h. Fluorescence images of i PC12 cellular uptake of NCs for 2 h and j differentiated PC12 cells with NCs under MF. k Quantitation of neurite length and percentage of cells with neurites in various conditions including NCs and MF treatment. Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparison test (n = 6, *p < 0.01, ***p < 0.001).
The synthesized GO with a mean size of ~2 μm was exfoliated from graphite, resulting in slow sedimentation of GO in water. After the formation of cookie structures on GO, the NCs were separated by centrifugation at 4000 r.p.m. to reduce their sizes, and the size distribution of the resulting NCs ranged from ~300–550 nm, as measured by DLS (Fig. 2d). Then, the BET method was utilized to investigate the pore structures through nitrogen absorption–desorption isotherms, which suggest that the surface area of the NCs was ~550 m2g−1; a pore size of 3.6 nm was determined by BJH analysis. The absorption–desorption curve exhibits typical Langmuir type IV characteristics with a hysteresis loop, which is the inherent property of mesoporous materials. Furthermore, the main hysteresis loop in the medium-pressure region indicates capillary condensation and multilayer adsorption in the mesopores. Compared to the NCs, both graphite and GO exhibited relatively low surface areas due to the nonporous structure (Fig. 2e and Supplementary Fig. S4).
NFG release from NCs actuated by MF
The magnetometry result of the NCs determined by a SQUID is given in Fig. 2f. The saturation magnetizations of the NCs were 0.001 and 0.4 e.m.u. g−1 at 300 K and 6 K, respectively. The weak magnetic properties were induced by defects on the graphene. Through adsorption, the loading capacity of NGF in the NCs is 290 µg g−1 NC. The high loading efficiency mostly reflects an affinity between NGF and carbon surfaces, which NCs possess in abundance. Furthermore, to evaluate the effects of the MF on NGF release, the NGF-loaded NCs (NGF@NCs) were subjected to an MF for 15 min at a power of 3.2 kW (1 MHz). A similar instrument developed by MagForce was approved for application in Europe to treat glioblastoma multiforme by local hyperthermia of iron oxide nanoparticles in brain tumors39. As shown in Fig. 2g, the amount of NGF released reached ~45% after 15 min of MF treatment. Without MF treatment, the natural release of NGF was ~14%. The main reason for the triggered NGF release is the electrostatic repulsive force between the protein and NCs. According to Lenz’s law, the direction of current in a conductor is induced by the external MF, which causes a MF that opposes the change that produced it. The magnetic flux causes the electromotive force in the conductive materials, and eddy currents are induced by the MF in closed loops within the stationary conductors40. As the NCs are a carbon-based conductive material, the MF potentially produces eddy currents in them. To investigate the MF effects on the NCs, the NCs were exposed to an MF for 15 min. After the MF treatment, the surface charge of the NCs estimated by a zeta potential analyzer changed to −26.3 mV from −6.5 mV due to the increased electrons on the particles. In addition, the NCs were slightly conductive, with a resistance of 1.64 kΩ cm−1, which is similar to that of graphene and its derivatives41,42, and the eddy current could be induced in the bulk NCs (compact NCs formed by hydraulic press) by MF to light up an LED (Supplementary Fig. S5a). To evaluate the eddy current, NCs were placed in DMEM supplemented with 10% FBS to mimic the physiological environment, and then subjected to an MF at various powers and distances with a frequency of 1 MHz. When subjected to an MF with a distance of 1 cm, currents of 0.78, 0.19, and 0.1 μA were measured at powers of 3.2, 1.92, and 0.32 kW, respectively (Supplementary Fig. S5b). Once the distance between the MF and NCs was increased to 2 cm, the induced currents were significantly decreased (Supplementary Fig. S5c).
Electromagnetized NCs for PC12 cytotoxicity and differentiation
The cytotoxicity of NCs with and without MF treatment was evaluated by incubating the NCs with PC12 cells, which were derived from a pheochromocytoma of a rat adrenal medulla line at different concentrations. Briefly, after coculturing the NCs and PC12 cells for 4 h, the cells were subjected to 5 and 15 min of MF; then, cell viability was examined at 24 h posttreatment. To monitor the heat induced by the eddy current, as shown in Supplementary Fig. S6, at 120 µg ml−1 NCs, the temperature of the solution was observed to be slightly changed (7 °C) under 35 °C, and the viability of the cells with 5 min of MF treatment was ~92%, indicating that the short MF treatment did not exhibit an obvious influence on the cells. When the treatment time was increased to 15 min, cell viability was maintained at 86%, suggesting that the long treatment caused slight cell death due to energy generation. The cellular uptake of the NCs was examined by flow cytometry, as shown in Fig. 2h, which reveals significant fluorescence of the NCs after 0.5, 1, and 2 h of incubation.
To evaluate the MF effect on NGF@NCs in vitro, the PC12 cells were treated with an MF for 15 min at 1 h postincubation of the particles. Without the MF, NGF@NCs were observed on the cells (Fig. 2i), and the PC12 cells exhibited a short neurite length (<15 µm) and a low differentiation percentage (<4%). However, once the PC12 cells were treated with the MF, the average neurite length and differentiation percentage increased to 245 µm and 18%, respectively, indicating effective PC12 cell differentiation when combining the NGF@NCs and MF (Fig. 2j). To clarify the MF and NGF effects, both the PC12 cell neurite length and number of cells with neurites were studied under various conditions, as shown in Fig. 2k. With the assistance of the MF, the average neurite lengths of the PC12 cells reached 46 and 116 µm for NCs and NGF@NCs, respectively. Generally, the neurite outgrowth of PC12 cells can be induced by NGF through activating the receptor tyrosine kinase, G protein-coupled receptors, and heterotrimeric G proteins. The results showed that the NCs were also able to induce PC12 cell differentiation without NGF; in addition, Fig. 2k (right panel) shows 8% of the cells with neurites under an MF. As documented in the literature, the electrical stimulation generated by locally charging objects is able to influence neuronal differentiation, biological processes, nerve regeneration processes, and neurite outgrowth through the upregulation of calcium signaling and phosphorylation of the cAMP-response element-binding pathway. Previous studies also documented that energy does not affect most cell types, but activates intracellular signaling to neurogenesis43.
As shown in Supplementary Fig. S7a, PC12 cells with and without attached NCs were placed together, and an MF was applied. PC12 cells attached to NCs exhibited clear enhancement of neurite outgrowth. Furthermore, to evaluate the effects of electromagnetized NCs on a biomaterial, NGF-NCs were coated on silk through layer-by-layer PLL/NC adsorption. As shown in Supplementary Fig. S7b, the surface of the silk displayed a rough morphology after NC coating, indicating a high density of NCs on the silk. Then, the PC12 cells were seeded on the substrate for 24 h of incubation, and the spherical morphology of PC12 cells was obtained because the cells were not differentiated with neurites (Supplementary Fig. S7c). While subjected to the MF for 15 min at a power of 3.2 kW, increased differentiation and neurite outgrowth were apparently observed on NC@silk (Supplementary Fig. S7d), and the synergistic effects of the NCs and MF were demonstrated on the PC12 cells (Supplementary Fig. S7e). This observation indicates that the electromagnetization of NC can be executed on a substrate and that cell fate can be manipulated, using an external physical energy source.
3D printing of NC@C
A light-curable bioink composed of 4-HBA, PU-EO-PO monomer, and Irgacure 819 (an initiator) could be polymerized by light with absorbance at a wavelength of 260 nm through 3D printing (Supplementary Fig. S8), and each curing layer in the DLP 3D printing process was ~50 µm. 4-HBA has a primary hydroxyl group at the end of a long alkyl chain, providing stretchable and flexible characteristics. To enhance the water retention and protein permeability, the EO and PO segments served as hydrophilic and hydrophobic parts in the elastomer, which was capable of incorporating water-soluble molecules. With the loading of NCs or red dye, the resulting printed NC-loaded elastomer (NC@elastomer) exhibited solid morphology and excellent stability in various architectures (Fig. 3a). The image in Fig. 3b depicts a dog-bone tensile specimen of the 3D printed elastomer. On the specimen, the appearance of the grid on the surface in the SEM image (the lower panel of Fig. 3b) resulted from the layer-by-layer printing and DLP light sources. The color and surface morphology of the sample would also be affected by adding NCs to the bioink, as shown in Fig. 3c, d. Once the concentration of NCs increased to 1%, large amounts of NCs were exposed on the surface via the 3D printing process (Fig. 3e). This phenomenon reflects the local curing of DLP 3D printing, where the particles between light-emitting and unexposed areas were fixed on the resulting matrix surface (Supplementary Fig. S9). However, when traditional molding was used to prepare the composite, the particles preferred to be embedded in the gel. Thus, by using this printing technology, the surface roughness can be controlled, which has been demonstrated to improve cell adhesion44.
The precision of the 3D printed matrix was evaluated by Zen Desk software by comparing the resulting 3D printed matrix and the original CAD image (Supplementary Fig. S10). The precision was 87.55% and 82.5% for the elastomer and NC@elastomer, respectively. Furthermore, the resulting NC@elastomer displayed an elongation of ~150% (Fig. 4a). While adding 10% of the PU-EO-PO monomer in the bioink, the elastic modulus could be ~2.5 times (Fig. 4b) because the aromatic urethane group of PU exhibits a strong intermolecular force. Moreover, by adding 1% NCs to the elastomer, the elastic modulus was increased to 0.34 MPa from 0.22 MPa (Fig. 4c and Supplementary Fig. S11). However, further addition of NCs to the bioink would lower the elasticity, so 1% NCs was used in the elastomer in the following drug/protein release, cell, and animal studies. Furthermore, to evaluate swelling behavior, the resulting NC@elastomer was placed in water and ethanol for 2 h. As shown in Fig. 4d, with the assistance of a partial hydrophilic segment of PU-EO-PO and NCs, the swelling ratio of the matrix was ~15% and 46% in water and ethanol, respectively. The NC@C could still maintain the elasticity and microchannels after 2 weeks of immersion (Fig. 4e). After swelling, protein penetration was investigated by immersing Texas Red™-conjugated ovalbumin (ThermoFisher, USA) in the NC@C; then, the cross section of the conduit was examined by confocal laser scanning microscopy (CLSM, Zeiss LSM800, Germany). As shown in Fig. 4f, a significant fluorescence signal of ovalbumin was observed on the surface of the conduits after 2 h of diffusion, where the green fluorescence was caused by the autofluorescence of the conduit. After increasing the time to 48 h, ovalbumin was detected in the whole conduit, and strong signals were also observed.
a 3D printed NC@elastomer with stretchable and elastic properties. The scale bar of the insert is 1 cm. b Tensile stress–strain diagram of NC@elastomer. c Tensile stress–strain diagram of 3D printed elastomer before and after adding NCs 4 h postcuring. d Swelling ratio of NC@C in water and ethanol (n = 6). e Images of NC@C with elastic properties (upper). Cross section of NC@C imaged using optical microscopy (lower). f Diffusion ability of ovalbumin (red) crossing into NC@C for 0, 8, and 24 h.
NGF penetration and release from NC@C under MF treatment
The protein penetration was potentially caused by the PU-EO-PO amphiphilic polymer chains and the NCs. Indeed, the NC amounts in the conduit reflected the protein-loading capacity of NC@C. As shown in Fig. 5a, the protein-loading capacity of 1%NC@C was approximately three times greater than that of the conduit alone. However, 1% NCs in the conduit did not obviously affect the loading capacity of small molecules when compared to that of the conduit alone, where doxorubicin (Dox) was used as a model drug. The mechanism was probably induced by the strong affinity between the carbon porous structure and the proteins, where the porous surface provided the binding sites to the proteins45. The external MF enhanced the penetration of Dox and ovalbumin into the NC@elastomer, which was monitored by CLSM from the top of the elastomer (Supplementary Fig. S12). The profiles of penetration depth under the influence of 15 min of MF treatment at the initial time point display several important features (Fig. 5b). (a) The penetration rate with an MF is much faster than that without an MF, as evident from the deep penetration of Dox and ovalbumin under MF treatment compared to that without. (b) The penetration rate of ovalbumin was slower than that of the small molecules, which could be understood in terms of the large molecular size of the protein. Similar triggered release results are displayed in Fig. 2g.
a Loading capacity of Dox and protein in the conduit, 0.1%NC@C and 1%NC@C (n = 6, mean ± s.d., **p < 0.01, t-test). b Diffusion distance of Dox and protein from NC@C at various times (n = 6, mean ± s.d.). c Cumulative release of Dox from NC@C (n = 6, mean ± s.d.). d Cumulative release of protein from NC@C (n = 6). The MF was applied at a power of 3.2 kW and frequency of 1 MHz for 15 min.
Since the MF is known to cause the local current to be mediated through the conductor of NCs, physical energy may enhance the chain flexibility of the elastomer, further aided by the affinity of the NCs to water, realizing the fast penetration of protein. Furthermore, the release patterns of Dox and NGF (a small and large model molecule, respectively) from NC@C under MF treatment were also investigated, as shown in Fig. 5c, d. For all the release profiles, there was an initial burst release, which manifested the rapid desorption of Dox and NGF from the surface of the conduit. Similar to the influence of the MF on penetration, the release rates were obviously increased while subjected to the MF due to the induced eddy current in the NCs. Although fast Dox and NGF release was obtained for the first hour to 3 days, the cumulative release of NGF reached only 60%, whereas the Dox release was ~100% due to the low diffusion rate of large molecules. Furthermore, Dox in conduits exhibited faster release than that in NC@C, likely due to the strong affinity of NC for Dox. These results suggest that the short MF exposure caused a permanent conformation change that sustained the release of the cargo for a few days.
Cell adhesion and PC12 differentiation on NC@C
The in vitro cell attachment and compatibility of NC@C were determined using fibroblast cells (HIG-82), SCs, and N2a incubated for 24 h. As shown in Fig. 6a, the three types of cells effectively attached and developed on the surface, where DAPI stained the nucleus and phalloidin stained F-actin. However, the conduit demonstrated weak cell adhesion. Compared to that on the cell culture dish, cell adhesion on the NC@C was ~80%, but cell adhesion on the conduit alone was only ~40%, as shown in Supplementary Fig. S13. This observation is reminiscent of the effect of roughness through particle exposure, which is the basis of the 3D printing composite process. As expected, 1% NCs increased the surface roughness of the conduits after the printing process, as shown in Supplementary Fig. S14. The profile roughness parameter (Ra) increased from 2.93 to 36.32 when adding 1% NCs to the bioinks during 3D printing. Having demonstrated cell adhesion, we followed ISO 10993-5 (tests for in vitro cytotoxicity) to determine the cell viability of the 3D printed materials before removal of unreacted agents. As shown in Fig. 6b, higher cell viability of the 3D printed materials was observed after 4 h postcuring in HIG-82 cells. The results indicated that polymers containing residual initiators and monomers would lead to considerable toxicity46. Therefore, the postcuring and removal of unreacted agents in the polymer by ethanol were apparently critical in the application of photocured NC@C. Figure 6c also reveals that the NCs slightly decreased the cell viability of the conduits, suggesting the low toxicity of the latter. Therefore, before NGF loading and animal studies were carried out, these agents were removed to mitigate toxicity.
a Morphologies of HIG-82, Schwann, and Neuro-2a cells on NC@C incubated for 1 day. DAPI stained the nucleus (red), and phalloidin stained F-actin (green). b Cell viability of HIG-82 cells incubated for 24 h with NC@C at various concentrations of NCs and postcuring times. c Cell viability of HIG-82, SC, and N2a cells incubated for 24 h with NC@C at various concentrations of NCs. d Fluorescence images of PC12 cells incubated with NGF-NC@C with and without MF treatment. e Quantitation of neurite length and f percentage of cells with neurites for PC12 cells in NGF@C, NC@C, and NGF-NC@C under MF. Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparison test (n = 6, ***p < 0.005, ****p < 0.001). g Fluorescence images of HIG-82 cells on NC@C with and without microchannels. The width and length of the microchannels are 0.7 and 10 mm, respectively. Quantitation of axon orientation for HIG-82 cells (right column).
For effective nerve outgrowth, differentiation should be triggered by an external physical cue and growth factors. For NC@C, we investigated the percentage of PC12 cells differentiated with neurites and the length of axonal outgrowth induced by NGF and MF (Supplementary Fig. S15a). Similar to a previous study, NCs treated with the MF impacted β-III-tubulin and the neuron-specific cytoskeletal protein in PC12 cells through the synergistic effect of NGF and electrical stimulation (Fig. 6d). As expected, the fluorescence images of PC12 cells with MF stimulation exhibited longer neurite extensions than those cells without treatment. To estimate the NGF (protein) activity after MF treatment, NGF was loaded on NCs in advance and released by applying an MF. The released NGF was then added to PC12 cells. As the results showed that the released NGF was still able to induce PC12 cell differentiation, indicating the activity of NGF (Fig. 6d). Statistical analysis of neurite length and neurite number over various treatments is also demonstrated in Fig. 6e, f. In the NGF-NC@C group, the average neurite length reached 40 µm, and >60% of the PC12 cells were differentiated after 15 min of MF treatment; for the PC12 cells without MF treatment, the average neurite length reached only 22 µm, and 31% of the cells were differentiated. The results confirmed the significant enhancement in the differentiation of PC12 cells under MF treatment through electromagnetic stimulation of the cells and intense NGF release. To further evaluate the effects of NC exposure on the conduits, the NCs were embedded in a gel to model PC12 cell differentiation. As shown in Supplementary Fig. S15b, c, while subjected to the MF, the NGF-NC-embedded gel could induce cell differentiation by triggering NGF release. However, compared to that for the exposed NC on the conduit, the neurite length or percentage of cells with neurites for the NGF-NC-embedded gel was decreased, indicating that the amplification of PC12 cells was stimulated by directly contacting the NCs to cells. Therefore, 3D printing to expose NCs on the conduits can significantly enhance cell differentiation. To ascertain the effects of the microchannels on cell orientation, HIG-82 cells were cultured on 3D printed conduits containing microgrooves with and without NCs. As shown in Fig. 6g, the cells on the conduit without channels had a random orientation. However, in the groups of conduits with channels, the HIG-82 cells were highly aligned after 4 days of incubation. This orientation was also quantified by a Gaussian distribution (oriented at a 0° basis angle), as shown in the right column of Fig. 6g, suggesting the effective improvement of in vitro cell orientation via these channels. Compared with the conduit with the channel (without NC), NC@C with the channel exhibited less orientation, which was potentially attributed to the surface roughness, facilitating cell adhesion47,48. To clarify the differences in the generated eddy currents in vitro and in vivo, the NCs were placed in DMEM supplemented with 10% FBS to mimic the cell culture conditions and biological tissue (animal muscle), respectively. The generated currents in vitro and in vivo were ~28 and ~38 μA, respectively (Supplementary Fig. S16). The differences may be attributed to the ions in the microenvironments.
Functional recovery of a regenerated sciatic nerve in vivo
To implement the spatial distribution of NGF for the promotion of axon outgrowth, 3D printed NC@C loaded with NGF was implanted after a peripheral nerve was transected 10 mm from the bifurcation point of the sciatic nerve in the popliteal fossa of SD rats. In the animal studies, five groups, including autograft, NC@C, NGF-NC@C, and NGF-NC@C+MF, were implanted in vivo in the sciatic nerve transection, where identical amounts of NGF (29 µg g−1 conduit) were applied in the NGF-loaded groups (Fig. 7a). The distance between the MF and the incision at the posterior of the left hind was maintained at 1 cm (Supplementary Fig. S17). The MF treatment was carried out for 15 min at a power of 3.2 kW and frequency of 1 MHz. The toe spreading of an injured hind paw and the SFI were measured 30 days postimplantation, which is an atypical walking footprint analysis of toe spreading. The SFI is 0 for healthy animals and −100 for animals with a transected sciatic nerve. After 1 month of treatment, a significantly wide toe spreading of the injured hind paw was observed in the NGF-NC@C+MF group, indicating excellent axonal regeneration (Fig. 7b, c), where 15 min of MF was locally applied at 4 days postimplantation at a power of 3.2 kW (1 MHz). After analysis, we observed that the SFI value of the NGF-NC@C+MF group was higher than those in the other groups. Furthermore, once sciatic nerve injury occurs without treatment, it causes atrophy of the gastrocnemius muscle (decreases in muscle weight and muscle fiber size), leading to a decreased weight ratio of the wet gastrocnemius muscles between the left and right sides. However, in the NGF-NC@C+MF group, the weight ratio of the gastrocnemius muscles was close to that in the autograft group, suggesting excellent recovery of the gastrocnemius muscles (Fig. 7d).
a Schematic illustration of the sciatic nerve defect sutured with the 3D printed conduit. Plantar view of the hind paws of rats with NGF-NC@C (lower picture). b The positions of PL, TS, and ITS. c SFI of each rat group calculated with TS, ITS, and PL (n = 5, statistical significance performed using one-way ANOVA with Tukey’s multiple comparison test (*p < 0.05, **p < 0.01)). d Weight ratio of the wet gastrocnemius muscles between the left (injured) and right (control) limbs. The insert represents an image of the left and right limbs in the NGF-NC@C+MF group (n = 5, statistical significance performed using one-way ANOVA with Tukey’s multiple comparison test (*p < 0.05)). e Size distributions of muscle fibers for normal, autograft, conduit, NGF-NC@C, and NGF-NC@C+MF. f TEM images of regenerated sciatic nerve in the cross section for autograft, NC@C, NGF-NC@C, and NGF-NC@C+MF. g Axon density of the regenerated sciatic nerve. h Number of myelin layers of the regenerated sciatic nerve (n = 5).
For peripheral nerve regeneration, the morphologies of atrophied muscle were used to estimate limb recovery. At 1 month postimplantation, the gastrocnemius muscles were harvested and stained by hematoxylin and eosin, as shown in Supplementary Fig. S18. Then, the diameter and distribution of the muscle fibers were calculated (Fig. 7e). The atrophied muscle in the conduit alone group demonstrated obvious degradation of muscles. Even though the weight ratio of the gastrocnemius muscles of the NGF-NC@C group was close to that of the autograft, weak recovery was observed in the muscle fibers, i.e., small fiber diameter. However, combining the MF treatment, the diameter of the muscle fibers was regained after nerve reinnervation and was close to that in the autograft (gold standard in nerve gap repair), as observed in the NGF-NC@C+MF group.
Regenerated myelinated nerve fibers
The myelinization process of SCs is a key factor in axonal regeneration after PNI because the developing nervous system is guided by a myelin sheath. Furthermore, distal SCs undergo atrophy due to the loss of axonal contact for a prolonged period, resulting in an inhibitory extracellular environment for nerve regeneration. Generally, stronger distal axons and myelination lead to better functional recovery for the injured nerve. As shown in Fig. 7f, the cross section of distal regenerative nerves evaluated by TEM at 1 month postimplantation suggests that the lamination of myelin sheathes of the regenerated nerve fibers in NGF-NC@C+MF was successful compared to that of the gold standard (autograft). However, in the NC@C and NGF-NC@C groups, the number of layers of myelin sheaths was smaller. After myelination analysis, the axon densities in each group were similar, but only NGF-NC@C+MF exhibited a similar number of myelin layers as that of the autograft (Fig. 7g, h). These findings indicated that NGF-NC@C+MF improved the diameter of the regenerative axon by controlling biocues and magnetoelectric stimulation.
To understand the growth and orientation of regenerated axons, the whole regenerated nerve from the proximal to distal section in the conduit was harvested, and stained with ß-III-tubulin and DAPI to track the regenerated axons and cell nuclei, respectively. Figure 8 displays a large area of CLSM images of immunohistochemically stained sciatic nerve defects from various treatments at 1 month postimplantation. Several conclusions could be drawn from these results. First, although the autograft group had a thick nerve, curled and random fiber-like morphologies of regenerated axons were observed because the direct surgical suture led to the mismatch of axons. Second, NGF-NC@C+MF exhibited a thicker nerve bundle than NGF-NC@C and NC@C, indicating that nerve growth was promoted by magnetoelectric stimulation and NGF release (inset picture). Third, a small distribution of axon orientation was detected in the conduit groups. This observation confirmed the effective regeneration of the MF treatment (i.e., magnetoelectric stimulation induced nerve differentiation mediated by NCs in the conduit, and NGF release enhanced cell growth). In addition, judging from the limb muscle weights and functions, the nerve recovery effects in NGF-NC@C+MF appear to be excellent. Electrophysiological analysis includes nerve conduction velocity (NCV) compound muscle action potential (CMAP) in Supplementary Fig. S19a, which is a classical electrophysiological method with diagnostic and prognostic value for evaluating PNI49,50. NCV and CMAP are highly influenced by axons and myelin, which are correlated with the maturation of regenerative nerve fibers17,51. A normal nerve was found to exhibit an NCV of 47.5 m s−1 and a CMAP of 46.2 mV. At 1 month postsurgery, the NGF-NC@C+MF group exhibited an NCV of 44.7 m s−1 and a CMAP of 44.9 mV, both of which were higher than those of the NGF-NC@C group (Supplementary Fig. S19b, c).
Sciatic nerve defects harvested from a autograft, b NC@C, c NGF-NC@C, and d NGF-NC@C+MF (n = 5). The inserted images show the optical images, and the bar charts show the orientation analysis. Direct axonal outgrowth followed the microchannels of the conduit. Green represents β-III-tubulin (axon), and blue represents nuclei. The orientation is from proximal to distal (n = 5).
To estimate the immune response for a long period, the autograft nerve and NC@C were harvested and compared after 2 months of surgery, where the numbers and distribution of macrophages were stained by CD68 antibody for tracking purposes (Supplementary Fig. S20a, b). The CLSM images revealed that there was no significant difference in the numbers of macrophages between the normal and NC@C groups, indicating low inflammation in the tissue. Furthermore, the weight changes of mice for autograft, conduit, NC@C, and NC@C+MF displayed almost identical patterns due to the biocompatibility of the implanted materials (Supplementary Fig. S20c). Next, six indices of liver as well as kidney functions, including albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, creatinine, and total bilirubin, and two strong predictors of all-cause mortality includingserum C-reactive protein and plasma D-dimers, were also evaluated after 60 days postimplantation of high-density polyene (control group), conduit, and NC@C (Supplementary Fig. S21). The differences between these groups were relatively small, implying the low toxicity of various treatments and safety of the materials. On the other hand, no severe immune response or tissue damage was observed once NC@C was implanted in muscle tissue, following the ISO 10993-6 test (tests for local effects after implantation, Supplementary Fig. S22). These results are consistent with previous studies exhibiting tolerable bioresponses for a long time.
In summary, we have developed a 3D printing method to prepare an NC-exposed conduit with stretchable and biocompatible characteristics. The NC, a nanocomposite composed of reduced GO, mesoporous silica, and a thin carbon layer, possesses superb attributes of magnetoelectric conversion, protein adsorption, and cell stimulation. After integrating the NCs into a bioink, the printed NC@C was able to carry large amounts of NGF with high efficiency and exhibited molecule/protein permeability. On-demand NGF release and cell stimulus were achieved in vitro using a high magnetoelectric treatment to effectively induce cell proliferation and differentiation, regaining the diameter of animal muscle fibers, as well as improving layers of myelin sheathes in vivo and directing the axon orientation. This printed NC@C with exposed particles, excellent mechanical properties, controllable NGF release, and magnetoelectric stimulus is expected to introduce a new approach for the regeneration of nerves and other tissues.
References
Fu, H. et al. Morphable 3D mesostructures and microelectronic devices by multistable buckling mechanics. Nat. Mater. 17, 268–276 (2018).
Yu, K., Xin, A., Du, H., Li, Y. & Wang, Q. Additive manufacturing of self-healing elastomers. NPG Asia Mater. 11, 7 (2019).
Liu, G., Zhao, Y., Wu, G. & Lu, J. Origami and 4D printing of elastomer-derived ceramic structures. Sci. Adv. 4, eaat0641 (2018).
Kim, Y., Parada, G. A., Liu, S. & Zhao, X. Ferromagnetic soft continuum robots. Sci. Robot. 4, eaax7329 (2019).
Cui, H. et al. Three-dimensional printing of piezoelectric materials with designed anisotropy and directional response. Nat. Mater. 18, 234–241 (2019).
Trenfield, S. J., Awad, A., Goyanes, A., Gaisford, S. & Basit, A. W. 3D Printing pharmaceuticals: drug development to frontline care. Trends Pharmacol. Sci. 39, 440–451 (2018).
Wang, X. et al. A 3D-printed scaffold with MoS2 nanosheets for tumor therapy and tissue regeneration. NPG Asia Mater. 9, e376 (2017).
Liu, X. et al. 3D printing of living responsive materials and devices. Adv. Mater. 30, 1704821 (2018).
Schaffner, M., Rühs, P. A., Coulter, F., Kilcher, S. & Studart, A. R. 3D printing of bacteria into functional complex materials. Sci. Adv. 3, eaao6804 (2017).
Yu, F. et al. Real-time manipulation of intestinal peristalsis by enteric-encapsulated magnetic nanoparticles & wearable 3D-printed devices. NPG Asia Mater. 11, 33 (2019).
Truby, R. L. et al. Soft somatosensitive actuators via embedded 3D printing. Adv. Mater. 30, e1706383 (2018).
Liang, K., Carmone, S., Brambilla, D. & Leroux, J. C. 3D printing of a wearable personalized oral delivery device: a first-in-human study. Sci. Adv. 4, eaat2544 (2018).
Zhao, Y. et al. Biomimetic composite scaffolds to manipulate stem cells for aiding rheumatoid arthritis management. Adv. Funct. Mater. 29, 1807860 (2019).
Johnson, B. N. et al. 3D printed anatomical nerve regeneration pathways. Adv. Funct. Mater. 25, 6205–6217 (2015).
Höke, A. Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans? Nat. Clin. Pract. Neurol. 2, 448–454 (2006).
Shahriari, D. et al. Scalable fabrication of porous microchannel nerve guidance scaffolds with complex ceometries. Adv. Mater. 31, 1902021 (2019).
Hsu, R. S. et al. Adaptable microporous hydrogels of propagating NGF-gradient by injectable building blocks for accelerated axonal outgrowth. Adv. Sci. 6, 1900520 (2019).
Van Huizen, A. V. et al. Weak magnetic fields alter stem cell-mediated growth. Sci. Adv. 5, eaau7201 (2019).
Gratwicke, J. et al. Bilateral deep brain stimulation of the nucleus basalis of meynert for parkinson disease dementia: A randomized clinical trial. JAMA Neurol. 75, 169–178 (2018).
Wu, C. H. et al. Trojan-horse nanotube on-command intracellular drug delivery. Nano Lett. 12, 5475–5480 (2012).
Lim, K. T. et al. Pulsed-electromagnetic-field-assisted reduced graphene oxide substrates for multidifferentiation of human mesenchymal stem cells. Adv. Healthc. Mater. 5, 2069–2079 (2016).
Yoo, J. et al. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy. Nat. Nanotechnol. 12, 1006–1014 (2017).
Ng, S., Noor, N. & Zheng, Z. Graphene-based two-dimensional Janus materials. NPG Asia Mater. 10, 217–237 (2018).
Lee, J. H. et al. Nondestructive real-time monitoring of enhanced stem cell differentiation using a graphene-Au hybrid nanoelectrode array. Adv. Mater. 30, e1802762 (2018).
Gao, T. et al. Transferable, transparent and functional polymer@graphene 2D objects. NPG Asia Mater. 6, e130 (2014).
Choi, S., Kim, S. & Kim, I. Ultrafast optical reduction of graphene oxide sheets on colorless polyimide film for wearable chemical sensors. NPG Asia Mater. 8, e315 (2016).
Zhao, G. et al. Reduced graphene oxide functionalized nanofibrous silk fibroin matrices for engineering excitable tissues. NPG Asia Mater. 10, 982–994 (2018).
Yang, H. et al. Printing hydrogels and elastomers in arbitrary sequence with strong adhesion. Adv. Funct. Mater. 29, 1901721 (2019).
Hung, K. C., Tseng, C. S., Dai, L. G. & Hsu, S. H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 83, 156–168 (2016).
Farokhi, M., Mottaghitalab, F., Fatahi, Y., Khademhosseini, A. & Kaplan, D. L. Overview of silk fibroin use in wound dressings. Trends Biotechnol. 36, 907–922 (2018).
Qian, Y. et al. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 9, 323 (2018).
Niu, Y. et al. Scaffolds from block polyurethanes based on poly(ɛ-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials 35, 4266–4277 (2014).
Huang, G. et al. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 117, 12764–12850 (2017).
Qing, H. et al. Heterostructured silk-nanofiber-reduced graphene oxide composite scaffold for SH-SY5Y cell alignment and differentiation. ACS Appl. Mater. Interfaces 10, 39228–39237 (2018).
Hummers, W. S. Jr & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1985).
Su, Y. L. et al. The penetrated delivery of drug and energy to tumors by lipo-graphene nanosponges for photolytic therapy. ACS Nano 10, 9420–9433 (2016).
Kudin, K. N. et al. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8, 36–40 (2008).
Zarrin, H., Higgins, D., Jun, Y., Chen, Z. & Fowler, M. Functionalized graphene oxide nanocomposite membrane for low humidity and high temperature proton exchange membrane fuel cells. J. Phys. Chem. C 115, 20774–20781 (2011).
Chiu-Lam, A. & Rinaldi, C. Nanoscale thermal phenomena in the vicinity of magnetic nanoparticles in alternating magnetic fields. Adv. Funct. Mater. 26, 3933 (2016).
Wang, P. et al. Assembly-induced thermogenesis of gold nanoparticles in the presence of alternating magnetic field for controllable drug release of hydrogel. Adv. Mater. 28, 10801–10808 (2016).
Pang, S., Hernandez, Y., Feng, X. & Müllen, K. Graphene as transparent electrode material for organic electronics. Adv. Mater. 23, 2779–2795 (2011).
Bonaccorso, F., Sun, Z., Hasan, T. & Ferrari, A. C. Graphene photonics and optoelectronics. Nat. Photonics 4, 611–622 (2010).
Lynch, K. J., Skalli, O. & Sabri, F. Growing neural PC-12 cell on crosslinked silica aerogels increases neurite extension in the presence of an electric field. J. Funct. Biomater. 9, 30 (2018).
Li, Y. et al. Antibody-modified reduced graphene oxide films with extreme sensitivity to circulating tumor cells. Adv. Mater. 27, 6848–6854 (2015).
Yang, Y. & Li, J. Lipid. protein and poly(NIPAM) coated mesoporous silica nanoparticles for biomedical applications. Adv. Colloid Interface Sci. 207, 155–163 (2014).
Chan, H. N. et al. Direct, one-step molding of 3D-printed structures for convenient fabrication of truly 3D PDMS microfluidic chips. Microfluid Nanofluid 19, 9–18 (2015).
Shuai, Y., Mao, C. & Yang, M. Protein nanofibril assemblies templated by graphene oxide nanosheets accelerate early cell adhesion and induce osteogenic differentiation of human mesenchymal stem cells. ACS Appl. Mater. Interfaces 10, 31988–31997 (2018).
Li, Y. et al. Antibody-modified reduced graphene oxide films with extreme sensitivity to circulating tumor cells. Adv. Mater. 2015, 6848–6854 (2015).
Raimondo, T. M. et al. Combined delivery of VEGF and IGF-1 promotes functional innervation inmice and improves muscle transplantation in rabbits. Biomaterials 216, 119246 (2019).
Wang, G. W., Yang, H., Wu, W. F., Zhang, P. & Wang, J. Y. Design and optimization of a biodegradable porous zein conduit using microtubes as a guide for rat sciatic nerve defect repair. Biomaterials 131, 145–159 (2017).
Das, S. et al. In vivo studies of silk based gold nano-composite conduits forfunctional peripheral nerve regeneration. Biomaterials 62, 66–75 (2015).
Acknowledgements
This work was financially supported by the Ministry of Science and Technology of the Republic of China, Taiwan under contracts MOST 109-2636-E-007-014 and MOST 108-2636-E-007-001, and by NTHU (107Q2512E1) in Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, JH., Hsu, HH., Hsu, RS. et al. 4D printing of stretchable nanocookie@conduit material hosting biocues and magnetoelectric stimulation for neurite sprouting. NPG Asia Mater 12, 61 (2020). https://doi.org/10.1038/s41427-020-00244-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-020-00244-1
This article is cited by
-
Magneto-mechanical effect of magnetic microhydrogel for improvement of magnetic neuro-stimulation
Nano Research (2023)
-
Research on imminent enlargements of smart materials and structures towards novel 4D printing (4DP: SMs-SSs)
The International Journal of Advanced Manufacturing Technology (2023)
-
Recent advances in 4D printing hydrogel for biological interfaces
International Journal of Material Forming (2023)
-
4D Printing in Biomedical Engineering: a State-of-the-Art Review of Technologies, Biomaterials, and Application
Regenerative Engineering and Translational Medicine (2023)
-
Industry news: the additive manufacturing of nerve conduits for the treatment of peripheral nerve injury
Bio-Design and Manufacturing (2022)