Abstract
Multifunctional organic/inorganic nanocomposites are highly attractive for effective biomedical applications. In this work, versatile types of self-destructible polysaccharide (dextran or pullulan) nanocomposites (denoted Au@PSa) with adjustable amounts of unlockable Au nanorods (Au NRs) were fabricated as highly efficient photothermal cancer therapy systems. Taking advantage of the acidic endosomes and high concentration of glutathione (GSH) present in cancer cells, the responsive self-destruction of Au@PSa nanocomposites could unlock the abundant encapsulated Au NRs within cells. Notably, the pullulan-based nanocomposites (denoted Au@Pul) demonstrated liver cell-targeting properties, which could enhance the therapeutic effects while minimizing side effects. The strong absorption of the unlocked Au NRs in the near-infrared region was utilized to examine the photothermal performance. The Au@PSa nanocomposites with a moderate amount of Au NRs in the matrix exhibited very impressive photothermal effects in tumor therapy, where the encapsulated Au NRs were unlocked in the tumor region to realize the observed high performance. The proposed system could be further extended, as the polysaccharides were functionalized with amino groups. The current work provides a facile strategy to construct flexible therapeutic platforms with responsive self-destruction features.
Similar content being viewed by others
Introduction
Cancer is still a deadly disease worldwide and is the leading cause of death1. A variety of therapeutic modalities, such as chemotherapy and photothermal therapy (PTT), have been developed to conquer the complexity of tumor and cancer metastasis2,3,4. As one of the most appealing strategies, PTT through the conversion of near-infrared (NIR) light into heat possesses the advantage of negligible damage to normal cells5,6,7. NIR light-absorbing plasmonic nanoparticles have been successfully employed for effective PTT, and most of these nanoparticles exploit passive targeting by the enhanced permeation and retention (EPR) effect8,9,10,11. Surface ligands for active cell targeting could further facilitate the preferential accumulation of nanomaterials12,13,14. In addition to targeting, stimuli-responsive nanomaterials that can distinguish between aberrant tumor regions and normal tissues to minimize side effects have been designed15,16,17,18,19,20. In addition, taking advantage of the properties of tumor intracellular environments, such as the presence of acidic endosomes (5.0–5.5) and a high concentration of glutathione (GSH, 2–10 mM), stimuli-responsive destructible nanomaterials are also ideal for achieving enhanced performance21,22.
Natural polysaccharides (PSa), including chitosan, dextran (Dex), and pullulan, are renewable materials with low toxicity, degradability, bioactivity, and excellent biocompatibility23,24. Due to the existence of abundant hydroxyl and other functional groups, PSa could be used to construct intriguing therapeutic platforms after appropriate modification24,25. Particularly, pullulan possesses an inherent liver-targeting property, and pullulan-based conjugates have been proven superior in cell-targeting biomedical applications26,27,28. Possessing tunable longitudinal surface plasmon resonance (LSPR), gold nanorods (Au NRs) demonstrate strong absorption in the desirable NIR region29,30. Versatile therapy platforms based on Au NRs have been extensively studied and demonstrate great potential in cancer theranostics31,32,33. It was reported that chitosan/Au NR nanospheres possess advantages of both chitosan and Au NRs in imaging-guided cancer therapy34,35. However, only one Au NR was loaded in each nanosphere, and this method was limited to chitosan. If more Au NRs could be loaded in one nanocapsule without severe aggregation to maintain a distinct LSPR peak in the NIR region, the relatively higher concentration of Au NRs would produce a better therapeutic performance. To explore diverse PSa types and enhance the photothermal performance, it is desirable to develop a general strategy to synthesize different kinds of PSa nanocomposites with a suitable loading amount of NRs.
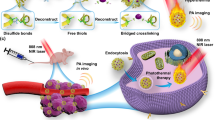
Herein, we propose a flexible strategy to construct versatile types of self-destructible PSa nanocomposites (Au@PSa) with adjustable amounts of unlockable Au NRs for efficient photothermal cancer therapy (Fig. 1). Water-soluble Dex and liver-targeting pullulan were explored as model systems. A variety of Au@PSa nanocomposites were readily achieved by the addition of Au NRs and the Schiff base crosslinking of aminated PSa. The acid-labile Schiff base bonds impart the resulting Au@PSa nanocomposites with the ability to self-destruct within cancer cells. In addition, the interaction between Au NRs and GSH could further accelerate the breakdown of the Au@PSa nanocomposites to unlock the abundant Au NRs36. Taking the enhanced photothermal effects from the large amount of unlocked Au NRs into account, the PTT performance of the Au@PSa nanocomposites is worth examination. Compared with the previous work in which more than one Au NR was encapsulated in mesoporous silica nanobeads37, diverse natural PSa provide not only a responsive self-destructible matrix but also inherent targeting properties, which could enhance the therapeutic effects while minimizing side effects. The responsive self-destruction and high-performance PTT of the Au@PSa nanocomposites were investigated in detail.
Materials and methods
Materials
Chloroauric acid, ascorbic acid, and glutaraldehyde (GA, 25 wt%) were obtained from Sinopharm Group Co. Ltd (China). Silver nitrate (99.8%), sodium borohydride (98%), cetyltrimethylammonium bromide (CTAB, 99%), 1,1′-carbonyldiimidazole (CDI, 97%), Dex (Mw ~23,800 g/mol), anhydrous dimethyl sulfoxide (DMSO), and ethylenediamine (ED, >98%) were obtained from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). Pullulan (Mw ~12,000 g/mol) was obtained from International Laboratory (USA). Ethylenediaminetetraacetic acid (EDTA) disodium salt was obtained from Beijing Chemical Works (Beijing, China). Fluorescein isothiocyanate isomer I (FITC, 95%) was obtained from Energy Chemical Co., Ltd (Shanghai, China). Fluorescein diacetate (FDA), propidium iodinate, (4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT), penicillin, and streptomycin were purchased from Sigma-Aldrich Chemical Co. HepG2 and C6 cell lines were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA).
Preparation of PSa-NH2
For the preparation of Dex-NH2, 0.5 g of Dex was dissolved in 3.5 mL of anhydrous DMSO, and 0.5 g of CDI in 1.5 mL of anhydrous DMSO was added dropwise. The resultant mixture was degassed by bubbling nitrogen through it for 10 min and kept at room temperature for 4 h. Then, 1.48 mL of ethylenediamine (ED) was added dropwise into the above solution under vigorous stirring. The reaction mixture was degassed by bubbling nitrogen through it for 10 min and stirred at room temperature for 48 h. After dialysis (MWCO 3500) against deionized water at room temperature for 48 h, the final product, Dex-NH2, was freeze dried. For the preparation of Pul-NH2, 1 g of pullulan was dissolved in 20 mL of anhydrous DMSO. Then, 1 g of CDI in 6 mL of anhydrous DMSO was added dropwise. The resultant mixture was kept at 37 °C for 6 h. Thereafter, 8.25 mL of ED was added, and the solution was stirred at 37 °C for 24 h. The final product, Pul-NH2, was dialyzed and freeze dried.
Preparation of Au@PSa nanocomposites
Au@PSa nanocomposites were prepared using the nonsolvent-aided counterion complexation method. Au NRs were synthesized using the typical seed-mediated growth method reported earlier33. For the preparation of Au@Dex nanocomposites, 1 mL of Dex-NH2 aqueous solution (0.3 mg/mL) was mixed with 1 mL of Au NR aqueous solution (0.059 mg/mL for Au@Dex-1, 0.12 mg/mL for Au@Dex-2 and 0.24 mg/mL for Au@Dex-3) and 1 mL of EDTA aqueous solution (1.2 mg/mL for Au@Dex-1 and Au@Dex-2 and 1.6 mg/mL for Au@Dex-3). The mixture was stirred vigorously at room temperature for 30 min. Then, 1.5 mL of ethanol was added dropwise under vigorous stirring, and the solution turned opalescent. Finally, 50 μL of GA solution (25 wt%) was added at room temperature. After 4 h, the resultant Au@Dex nanocomposites were collected by centrifugation and washed with deionized water four times to remove CTAB and any residual reactants.
For the preparation of Au@Pul nanocomposites, 2 mL of Pul-NH2 solution (0.5 mg/mL) was mixed with 2 mL of Au NR solution (0.059 mg/mL for Au@Pul-1, 0.12 mg/mL for Au@Pul-2, and 0.24 mg/mL for Au@Pul-3) and 2 mL of EDTA solution (1.5 mg/mL for Au@Pul-1 and 1.0 mg/mL for Au@Pul-2 and Au@Pul-3). The final mixture was stirred vigorously at room temperature for 30 min. Then, 3 mL of ethanol was added dropwise under vigorous stirring. Finally, 100 μL of GA solution (25 wt%) was added. After 4 h, the Au@Pul nanocomposites were collected by centrifugation.
Responsive self-destruction of the Au@PSa nanocomposites
The destruction of nanocomposites was carried out in a pH 5.0 solution. First, 25 μL of a Au@PSa suspension (2 mg/mL) was added into 975 μL of a pH 5.0 solution in the presence or absence of 10 mM GSH and then incubated at 37 °C for 24 or 48 h.
Cellular internalization
Cellular internalization was evaluated in HepG2 and C6 cell lines by flow cytometry, fluorescence microscopy, and confocal laser scanning microscopy (CLSM). FITC was used to conjugate the Au@PSa nanocomposite for visualization. The synthetic protocol was based on a method published elsewhere38. First, 40 μL of FITC in DMSO solution (1 mg/mL) was added to 20 mL of Au@Dex-2 or Au@Pul-2 solution (0.05 mg/mL, pH 11), and the solution was then stirred for 1 h at 40 °C. The FITC-labeled Au@Dex-2 and Au@Pul-2 nanocomposites were collected by centrifugation, washed with deionized water five times and dried under vacuum. The detailed procedures are shown in the Supporting Information.
Bio-TEM observation of Au@PSa-treated HepG2 cells
Bio-transmission electron microscope (Bio-TEM) was carried out to observe the distribution of Au@PSa in treated HepG2 cells following the previously reported procedure39. The details are shown in the Supporting Information.
In vitro cytotoxicity assay
The cytotoxicity of Au@PSa was evaluated by the MTT viability assay in HepG2 cell lines40. The detailed procedures are shown in the Supporting Information.
Photothermal effect of Au@PSa
Three milliliters of Au@PSa solution (200 μg/mL) in a quartz cuvette was irradiated by an 808 nm laser (2 W/cm, 5 min), and the temperature was recorded by an IR thermal camera. HepG2 cells were cultured with Au@PSa at various mass concentrations for in vitro photothermal experiments. For in vivo NIR thermal imaging, tumor-bearing nude mice were injected with 150 μL of Au@Pul-2 solution (3 mg/mL). The detailed procedures are shown in the Supporting Information.
In vivo PTT
HepG2 tumor-bearing nude mice were treated by injection of 150 μL of phosphate-buffered saline (PBS) (control group), Au@Pul-2 (3 mg/mL), and Au@Pul-2 (3 mg/mL) with irradiation by an NIR laser (PTT group, 2 W/cm, 10 min). The control group and PTT group were irradiated by an 808 nm laser (2 W/cm2, 10 min) only once after injection. Hematoxylin and eosin (H&E) staining was carried out according to the standard protocols described in the previous work41. The detailed procedures are shown in the Supporting Information.
Statistical analysis
All experiments were repeated at least three times. Data are presented as the mean ± standard deviation. Statistical significance (p < 0.05) was evaluated by a t test when two groups of samples were compared, and statistical significance was set at p < 0.05.
Results and discussion
Synthesis and characterization of the Au@PSa nanocomposites
For the synthesis of the Au@PSa nanocomposites, the hydroxyl groups of PSa (Dex or pullulan) were first activated by CDI and reacted with ED to produce PSa-NH2 with primary amino groups (Fig. 1)26,42. Typical proton nuclear magnetic resonance (1H NMR) spectra of Dex-NH2 and Pul-NH2 are shown in Figure S1 in the Supporting Information. Based on the 1H NMR spectra, amino groups were successfully conjugated to the glucose units of Dex and pullulan. Then, Au@PSa nanocomposites were synthesized by employing PSa-NH2, Au NRs with an LSPR peak at 780 nm and EDTA as the starting materials. EDTA attached to the Au NR surfaces through electrostatic interactions with the stabilizer CTAB. With the addition of ethanol, which is a nonsolvent for both PSa and EDTA, counterion condensation of cationic PSa-NH2 and anionic EDTA occurred around the Au NRs as the nucleation center35,37. After the formation of Schiff base bonds between the amino groups of PSa-NH2 and the aldehyde groups of GA, EDTA was removed through centrifugation, and Au NRs were encapsulated in the PSa-NH2 matrix to form Au@PSa nanocomposites.
As displayed in Fig. 2, versatile Au@PSa nanocomposites with adjustable amounts of Au NRs were readily achieved by modulating the concentrations of Au NRs, PSa-NH2, and EDTA. Both Au@Pul and Au@Dex nanocomposites with different amounts of encapsulated Au NRs, denoted Au@Dex-1, Au@Dex-2, and Au@Dex-3 (Fig. 2a) and Au@Pul-1, Au@Pul-2, and Au@Pul-3 (Fig. 2b), were fabricated successfully. The sizes of the Au@Dex nanocomposites were comparable (89 ± 6, 96 ± 4, and 91 ± 15 nm, respectively), while the Au@Pul counterparts were slightly larger (117 ± 22, 113 ± 18, and 118 ± 21 nm, respectively). The surface potentials of all the Au@PSa nanocomposites were approximately 30 mV (Figure S2, Supporting Information). As displayed in Fig. 2c, the LSPR absorption peaks of Au@Dex-1, Au@Dex-2, and Au@Dex-3 were 780, 767, and 707 nm, respectively. In other words, with an increasing amount of encapsulated Au NRs, an obvious blueshift of the LSPR peak occurred. Notably, the LSPR peak of Au@Dex-3 was almost distorted due to its largest degree of encapsulated Au NR aggregation, which was observed and confirmed by a discrete dipole approximation calculation in the previous report43. The same tendency was observed in the case of the Au@Pul nanocomposites from Au@Pul-1 to Au@Pul-3, where the LSPR absorption peak moved from 760 to 660 nm (Fig. 2e). The encapsulation of more Au NRs in the nanocomposites caused the absorption spectrum to approach that of spherical Au nanoparticles, and a blueshift occurred. The photothermal properties of the Au@PSa nanocomposites were further investigated, where Au@PSa suspensions with a mass concentration of 200 μg/mL were irradiated by an NIR laser at 808 nm (Fig. 2d, f). At the same mass concentration, Au@Dex-2 and Au@Pul-2 demonstrated higher temperatures due to their suitable absorption intensities and loading amounts of Au NRs. Thus, the Au@Dex-2 and Au@Pul-2 nanocomposites with moderate amounts of Au NRs were selected as the model system for the following studies. The weight ratio of Au NRs encapsulated in Au@Pul-2 and Au@Dex-2 was comparable (~40%) based on thermogravimetric analysis (Figure S3, Supporting Information).
Responsive destruction of the Au@PSa nanocomposites
As a responsive therapeutic platform, the self-destruction of the nanocomposites in an aberrant tumor intracellular environment would help to enhance the photothermal effect44. Fig. 3a illustrates the morphological evolution of Au@Pul-2 when incubated at pH 5.0 to simulate the acidic endosomes and lysosomes (5.0–5.5). The high concentration of GSH present within cancer cells was also considered to unlock the Au NRs by accelerating the destruction of the Au@PSa nanocomposites. After incubation at pH 5.0 for 1 day, the matrix of the Au@Pul-2 nanocomposite seemed to dissolve and be destroyed, probably due to the breakage of the Schiff base bonds. After 2 days, more of the matrix dissolved, while looser complexes of pullulan-Au were observed. When GSH was added to the acidic solution, the Au@Pul-2 nanocomposite further broke down into discrete Au NRs. The Au@Dex-2 nanocomposite underwent similar decomposition processes, and more readily unlocked Au NRs appeared in the presence of GSH (Figure S4, Supporting Information). The strong interaction between the Au NRs and thiol groups of GSH contributed to the unlocking of Au NRs from the matrix45,46, which in turn accelerated the responsive destruction of the Au@PSa nanocomposites. The discrete Au NRs unlocked from the nanocomposites were anticipated to enhance the photothermal effect because of their favorable optical properties. Figure 3b displays the temperature elevation of the Au@Pul-2 suspension before and after self-destruction. It is evident that the temperature elevation of the self-destructed sample at pH 5.0 (42 °C) after irradiation is higher than the original one (39 °C). In the presence of GSH, the temperature elevation further increased to 46 °C, confirming the enhanced photothermal effect of the unlocked Au NRs from the Au@Pul-2 nanocomposite. Therefore, GSH could pull the encapsulated Au NRs out of the nanocomposites through the formation of Au-S bonds so that the LSPR peaks of the self-destructed sample redshifted to result in an enhanced photothermal effect.
Cellular internalization of the Au@PSa nanocomposites
Pullulan is considered to possess a specific affinity for hepatoma cells with asialoglycoprotein receptor (ASGPR) overexpression47. In this study, the anticipated hepatoma-targeting property of the Au@Pul nanocomposite was verified by comparison of cellular internalization of Au@Dex-2 and Au@Pul-2 in hepatoma HepG2 cells. Glioma C6 cells without ASGPR were employed as the control. As shown in Fig. 4a, both FITC-labeled Au@Dex-2 and Au@Pul-2 were internalized by C6 and HepG2 cells after 4 h of incubation. It was observed that the green fluorescence signal from the Au@Pul-2-treated HepG2 cells was significantly stronger than that of the other groups. To quantitatively compare the cellular uptake, flow cytometry was performed to analyze the internalization ratio. In the C6 cell line, Au@Dex-2 and Au@Pul-2 demonstrated respective internalization ratios of 21% and 26%, which are relatively low and comparable to one another. In contrast, Au@Pul-2 displayed a much higher internalization ratio (90%) than Au@Dex-2 (24%) in the HepG2 cell line, proving that the Au@Pul-2 nanocomposite preserves the excellent hepatoma-targeting ability of pullulan. Therefore, the Au@Pul-2 nanocomposite was assumed to facilitate targeted cancer therapy.
The internalization of Au@Dex-2 and Au@Pul-2 in HepG2 cells was also monitored with real-time CLSM (Fig. 4b). The nucleus was stained with DAPI (4',6-diamidino-2-phenylindole, blue fluorescence), while FITC-labeled Au@Dex-2 and Au@Pul-2 were visualized in green. The signals of the Au@Dex-2 nanocomposite were weak after 0.5–4 h of incubation, while the Au@Pul-2 nanocomposite was readily internalized in HepG2 cells. An obvious time-dependent trend was observed. After 4 h of incubation, a large portion of the Au@Pul-2 nanocomposite accumulated around the nuclei, exhibiting strong fluorescence signals, while little fluorescence was observed for Au@Dex-2, indicating that Au@Pul-2 entered the cells much faster. Considering the fact that the particle sizes and surface potentials of Au@Dex-2 and Au@Pul-2 are similar (Fig. 2a, b and Figure S2, Supporting Information), the dramatic difference in cellular uptake was not caused by the surface morphology or size. These findings further confirmed the specific affinity of Au@Pul-2 for hepatoma cells. In addition, the internalization of Au@Pul-2 was believed to undergo receptor-mediated endocytosis through binding with overexpressed ASGPR48.
The internalized amount of Au@Dex-2 and Au@Pul-2 in HepG2 cells was quantitatively analyzed by inductively coupled plasma mass spectrometry (ICP-MS). As shown in Fig. 5a, a significant difference was found between the Au contents of the HepG2 cells treated with Au@Dex-2 and Au@Pul-2, also suggesting that Au@Pul-2 could efficiently enter HepG2 cells. Furthermore, the Au@Dex-2-treated and Au@Pul-2-treated HepG2 cells were sectioned to visualize the distribution of the Au@PSa nanocomposites. Judging from the Bio-TEM images of sectioned HepG2 cells, both Au@PSa nanocomposites were mainly localized in the cytoplasm (Fig. 5b, c), and larger amounts of Au@Pul-2 over Au@Dex-2 were found in the cell, in agreement with the ICP-MS results (Fig. 5a).
In vitro photothermal effect of the Au@PSa nanocomposites
The strong LSPR absorption and self-destruction features of Au@Pul-2 and Au@Dex-2 motivated us to exploit their photothermal effects. The weight ratio of encapsulated Au NRs was comparable between Au@Pul-2 and Au@Dex-2 based on the results of thermogravimetric analysis (Figure S4, Supporting Information). Dynamic light scattering measurements revealed that the hydrodynamic sizes of Au@Dex-2 and Au@Pul-2 in the culture medium supplemented with 10% fetal bovine serum were comparable (approximately 175 nm) and quite stable during incubation (Fig. 6a). Moreover, the surface charges of Au@Dex-2 and Au@Pul-2 were also equivalent (Figure S2, Supporting Information). Therefore, it is reasonable to compare the photothermal properties of these two kinds of Au@PSa nanocomposites. As demonstrated in Fig. 2d, f, the temperature elevation of the Au@Dex-2 and Au@Pul-2 suspensions at the same concentration of 200 μg/mL were also comparable (approximately 38 °C and 39 °C, respectively), suggesting that the nanocomposites have similar photothermal effects in solution.
a Particle sizes of Au@Dex-2 and Au@Pul-2 in medium supplemented with 10% fetal bovine serum (FBS) (mean ± SD, n = 3). b Cell viability assay of HepG2 cells treated with Au@Dex-2 and Au@Pul-2 at various concentrations without and with NIR irradiation (mean ± SD, n = 6; *significant difference, P < 0.05). c Representative fluorescence images of FDA-PI-stained HepG2 cells under irradiation and treatment with Au@Pul-2 (30 μg/mL) in the absence and presence of irradiation at 808 nm
To investigate the in vitro photothermal properties, an MTT assay was carried out to evaluate the cell viability under treatment by Au@Dex-2 and Au@Pul-2 in the HepG2 cell line in the absence or presence of NIR irradiation. As shown in Fig. 6b, the cell viabilities mediated by Au@Dex-2 and Au@Pul-2 were observed to change slightly with increasing concentration without NIR irradiation. At the high concentration of 60 μg/mL, the viability of the HepG2 cells was still above 80%, consistent with the good biocompatibilities of Dex and pullulan. Given the impressive photothermal performances (Fig. 2d, f), Au@Dex-2 and Au@Pul-2 are considered promising candidates for efficiently killing cancer cells under NIR irradiation. A preliminary in vitro experiment of the viability of HepG2 cells under NIR irradiation was quantitatively conducted. As shown in Fig. 6b, the cell viabilities under NIR irradiation decreased, unlike the ones without irradiation. Compared with the Au@Dex-2-treated cells, the cells treated with Au@Pul-2 exhibited a substantially lower viability at the same concentration. In particular, significant differences between the two groups were observed when the concentration was 30 or 60 μg/mL. The cell viability of HepG2 cells was as low as 19% when the concentration of Au@Pul-2 was 60 μg/mL. These results confirmed the superior photothermal effect of Au@Pul-2, which might be caused more by its effective internalization and the specific affinity of Au@Pul-2 for HepG2 cells. After the HepG2 cells treated with the Au@Pul-2 nanocomposite were exposed to the NIR laser, considerable cell death was observed in the live/dead staining images with FDA and PI, where most HepG2 cells remained viable under laser irradiation only or when treated by Au@Pul-2 without irradiation (Fig. 6c). These intuitive observations further suggest the excellent targeting ability and photothermal characteristics of the Au@Pul-2 nanocomposite for killing HepG2 cells.
In vivo PTT
To validate the feasibility of Au@Pul-2 for application in in vivo PTT, tumor-bearing nude mice were injected with Au@Pul-2 or PBS (control), and then the tumors were irradiated by an 808 nm laser (2 W/cm2). The real-time IR thermal images with intuitive temperature changes reveal the photothermal effect of Au@Pul-2 (Fig. 7a). As displayed in Fig. 7b, the corresponding temperature elevation in the Au@Pul-2 group increased to approximately 24 °C, which could be utilized to suppress tumor growth. The control group showed negligible changes, in accordance with the photographs and temperature variation of the tumor region.
a Infrared thermal images and (b) temperature variation in HepG2 tumor-bearing mice after injection with PBS (control) or Au@Pul-2 in the presence of 808 nm irradiation (2 W/cm2). c Time-dependent growth curves, weights and photographs of tumors after the different treatments. H&E staining images of the tumors (d) and (e) major organs (hear, liver, spleen, kidney, and lung) of mice. Scale bars: 50 μm
Encouraged by the above effective photothermal performances, we verified the hypothesis that Au@Pul-2 could be employed for in vivo PTT with satisfactory anticancer effectiveness. Liver tumor-bearing nude mice were randomly divided into three groups for different administrations: control (PBS only), Au@Pul-2, and Au@Pul-2 + NIR (PTT group). The volumes of the tumors were recorded every 2 days to monitor the growth rate. After 10 days, all the mice were euthanized, and the tumors of the different groups were weighed and imaged (Fig. 7c). The tumors of the control group were found to grow very fast, manifesting the typical high-proliferation-rate behavior of liver cancer. Tumor growth in the Au@Pul-2 group was not much different than that in the control group, consistent with the low cytotoxicity of Au@Pul-2 (Fig. 6b). However, for the PTT group, apparent tumor growth inhibition was observed, in accordance with the excellent photothermal performance of Au@Pul-2 (Fig. 6). Judging from the cellular internalization and in vitro photothermal results (Figs. 4–6), the Au@Pul-2 nanocomposite possesses an excellent targeting capability for hepatoma cells, which might facilitate the accumulation and retention in the region of hepatoma to enhance the PTT effectiveness. Remarkably, the tumors of two mice in the PTT group finally disappeared after the treatment, suggesting the superiority of PTT with the self-destructible Au@Pul-2 nanocomposite. The pathological test revealed by H&E staining of tumor slices further confirmed the therapeutic effect (Fig. 7d). Massive shrinking and fragmented cell nuclei were observed in the PTT group, while negligible tumor cell apoptosis was found in the control and Au@Pul-2 groups, providing evidence for safe PTT by the Au@Pul-2 nanoparticles with a moderate dose of irradiation.
Moreover, histological analysis of the major organs, including the heart, liver, spleen, kidney, and lung, was also conducted to evaluate the side effects of PTT. As displayed in Fig. 7e, the images of the H&E-stained major organs in the PTT group did not present any abnormalities compared with those of the control group. The body weight of all the three groups of mice increased during the administration period and displayed no distinct differences (Figure S5, Supporting Information), validating the compatibility and feasibility of Au@Pul-2 for PTT. Therefore, Au@Pul-2-mediated PTT would be impactful in the treatment of high-proliferation-rate HepG2 tumors.
Precise and accurate imaging during the therapeutic processes could provide valuable feedback for obtaining the best antitumor effect49. Taking advantage of their NIR absorption, Au NRs could realize PA imaging, an emerging imaging modality that offers sensitive signals of tumors located within a few centimeters50. The feasibility of Au@Pul-2 for PA imaging was also testified in vitro and in vivo. As the concentration of Au@Pul-2 increased, the corresponding intensity of the PA signals increased, and stronger PA images were accordingly observed (Figure S6a). After Au@Pul-2 suspensions were injected into HepG2 tumor-bearing nude mice, remarkably enhanced PA signals compared with those of the original tumor region were obtained (Figure S6b), confirming the tremendous potential of Au@Pul-2 for PA imaging-guided PTT.
Conclusions
In summary, versatile types of Au@PSa nanocomposites (Au@Pul and Au@Dex) consisting of adjustable amounts of Au NRs and pullulan or Dex were prepared successfully through a facile strategy. In this regard, the system could be further extended, as the PSa were functionalized with amino groups. Au@PSa nanocomposites possess a responsive self-destruction characteristic that enables unlocking of the Au NRs from the matrix to achieve better photoperformance. Because of the good biocompatibility and liver-targeting properties of pullulan, the Au@Pul nanocomposites exhibited an excellent affinity for HepG2 cells and superior in vivo photothermal performance. It is worth mentioning that the abundant hydroxyl groups of the PSa could be utilized to introduce more functionality, while the versatile Au@PSa matrix could be tailored to other biomedical applications. Therefore, the present study not only offers a general approach for the synthesis of Au@PSa nanocomposites but also opens a new avenue for the rational design of multifunctional therapeutic platforms.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Cleary, A. S., Leonard, T. L., Gestl, S. A. & Gunther, E. J. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature 508, 113–117 (2014).
Xu, F. J. Versatile types of hydroxyl-rich polycationic systems via O-heterocyclic ring-opening reactions: from strategic design to nucleic acid delivery application. Prog. Polym. Sci. 78, 56–91 (2018).
Yang, Y. et al. Core-shell and co-doped nanoscale metal-organic particles (NMOPs) obtained via post-synthesis cation exchange for multimodal imaging and synergistic thermo-radiotherapy. NPG Asia Mater. 9, e344 (2017).
Lyu, Y. et al. Dendronized semiconducting polymer as photothermal nanocarrier for remote activation of gene expression. Angew. Chem. Int. Ed. 129, 9283–9287 (2017).
Zhu, H. et al. Regulating near-infrared photodynamic properties of semiconducting polymer nanotheranostics for optimized cancer therapy. ACS Nano 11, 8998–9009 (2017).
Thapa, R. K., Byeon, J. H., Ku, S. K., Yong, C. S. & Kim, J. O. Easy on-demand self-assembly of lateral nanodimensional hybrid graphene oxide flakes for near-infrared-induced chemothermal therapy. NPG Asia Mater. 9, e416 (2017).
Lim, E. K. et al. Nanomaterials for theranostics: recent advances and future challenges. Chem. Rev. 115, 327–394 (2015).
Fan, W., Yung, B., Huang, P. & Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 117, 13566–13638 (2017).
Song, L., Zhao, N. & Xu, F. J. Hydroxyl-rich polycation brushed multifunctional rare-earth-gold core-shell nanorods for versatile therapy platforms. Adv. Funct. Mater. 27, 1701255 (2017).
Farrell, D. et al. Recent advances from the national cancer institute alliance for nanotechnology in cancer. ACS Nano 4, 589–594 (2010).
Ulbrich, K., Holá, K., Šubr, V., Bakandritsos, A. & Tuček, J. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 116, 5338–5431 (2016).
Dan, P. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Friden, P. M. et al. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Nat. Acad. Sci. USA 88, 4771–4775 (1991).
Dai, Y., Xu, C., Sun, X. & Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumor microenvironment. Chem. Soc. Rev. 46, 3830–3852 (2017).
Such, G. K., Yan, Y., Johnston, A. P., Gunawan, S. T. & Caruso, F. Interfacing materials science and biology for drug carrier design. Adv. Mater. 27, 2278–2297 (2015).
Nowag, S. & Haag, R. pH-responsive micro- and nanocarrier systems. Angew. Chem. Int. Ed. 53, 49–51 (2014).
Wu, X., Li, Y., Lin, C., Hu, X. Y. & Wang, L. GSH- and pH-responsive drug delivery system constructed by water-soluble pillar[5]arene and lysine derivative for controllable drug release. Chem. Commun. 51, 6832–6835 (2015).
Danhier, F., Feron, O. & Préat, V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control Rel. 148, 135–146 (2010).
Hu, Y. W. et al. Selective redox-responsive drug release in tumor cells mediated by chitosan based glycolipid-like nanocarrier. J. Control Rel. 206, 91–100 (2015).
Zhao, Y. et al. A preloaded amorphous calcium carbonate/doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew. Chem. Int. Ed. 54, 919–922 (2015).
Zhang, Q., Shen, C., Zhao, N. & Xu, F. J. Redox-responsive and drug-embedded silica nanoparticles with unique self-destruction features for efficient gene/drug codelivery. Adv. Funct. Mater. 27, 1606229 (2017).
Mizrahy, S. & Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 41, 2623–2640 (2012).
Hu, Y., Li, Y. & Xu, F. J. Versatile functionalization of polysaccharides via polymer grafts: from design to biomedical applications. Acc. Chem. Res. 50, 281–292 (2017).
Wang, R. et al. Versatile functionalization of amylopectin for effective biomedical applications. Sci. China Chem. 58, 1461–1470 (2015).
Ren, Y. et al. Effective codelivery of lncRNA and pDNA by pullulan-based nanovectors for promising therapy of hepatocellular carcinoma. Adv. Funct. Mater. 26, 7314–7325 (2016).
Yang, X. C., Niu, Y. L., Zhao, N., Mao, C. & Xu, F. J. A biocleavable pullulan-based vector via ATRP for liver cell-targeting gene delivery. Biomaterials 35, 3873–3884 (2014).
Huang, Y., Hu, H., Li, R. Q., Yu, B., & Xu, F. J . in Versatile types of MRI-visible cationic nanoparticles involving pullulan polysaccharides for multifunctional gene carriers ACS Appl. Mater. Interfaces. 3919–3927 2016.
Dreaden, E. C., Alkilany, A. M., Huang, X., Murphy, C. J. & El-Sayed, M. A. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41, 2740–2779 (2012).
Yang, X., Yang, M., Pang, B., Vara, M. & Xia, Y. Gold nanomaterials at work in biomedicine. Chem. Rev. 115, 10410–10488 (2015).
Zhao, N. et al. Hierarchical nanohybrids of gold nanorods and PGMA-based polycations for multifunctional theranostics. Adv. Funct. Mater. 26, 5848–5861 (2016).
Duan, S., Yang, Y., Zhang, C., Zhao, N. & Xu, F. J. NIR-responsive polycationic gatekeeper-cloaked hetero-nanoparticles for multimodal imaging-guided triple-combination therapy of cancer. Small 13, 1603133 (2017).
Yan, P. et al. Polycation-functionalized gold nanoparticles with different morphologies for superior gene transfection. Nanoscale 7, 5281–5291 (2015).
Guo, R. et al. Multifunctional nanocarriers for cell imaging, drug delivery, and near-IR photothermal therapy. Langmuir 26, 5428–5434 (2010).
Chen, R. et al. Near-IR-triggered photothermal/photodynamic dual-modality therapy system via chitosan hybrid nanospheres. Biomaterials 34, 8314–8322 (2013).
Li, Q., Chen, M., Chen, D. & Wu, L. One-pot synthesis of diphenylalanine-based hybrid nanospheres for controllable pH- and GSH-responsive delivery of drugs. Chem. Mater. 28, 6584–6590 (2016).
Chen, P. J. et al. A novel multifunctional nano-platform with enhanced anti-cancer and photoacoustic imaging modalities using gold-nanorod-filled silica nanobeads. Chem. Commun. 49, 892–894 (2013).
Schreiber, A. B. & Haimovich, J. Quantitative fluorometric assay for detection and characterization of Fc receptors. Method. Enzymol. 93, 147–155 (1983).
Huo, S. et al. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 8, 5852–5862 (2014).
Yan, P. et al. A facile strategy to functionalize gold nanorods with polycation brushes for biomedical applications. Acta Biomater. 10, 3786–3794 (2014).
Liu, Y. et al. Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 25, 1353–1359 (2013).
Song, H. Q. et al. A general strategy to prepare different types of polysaccharide-graft-poly(aspartic acid) as degradable gene carriers. Acta Biomater. 12, 156–165 (2015).
Jain, P. K., Eustis, S. & El-Sayed, M. A. Plasmon coupling in nanorod assemblies: optical absorption, discrete dipole approximation simulation, and exciton-coupling model. J. Phys. Chem. B 110, 18243–18253 (2016).
Bian, S. et al. One-pot synthesis of redox-labile polymer capsules via emulsion droplet-mediated precipitation polymerization. Chem. Mater. 27, 1262–1268 (2015).
Luo, Z. & Zhang, S. Designer nanomaterials using chiral self-assembling peptide systems and their emerging benefit for society. Chem. Soc. Rev. 41, 4736–4754 (2012).
Erathodiyil, N. & Ying, J. Y. Functionalization of inorganic nanoparticles for bioimaging applications. Acc. Chem. Res. 44, 925–935 (2011).
Kaneo, Y., Tanaka, T., Nakano, T. & Yamaguchi, Y. Evidence for receptor-mediated hepatic uptake of pullulan in rats. J. Control Rel. 70, 365–373 (2001).
Wang, H. et al. Dual-responsive nanoparticles based on oxidized pullulan and a disulfide-containing poly(beta-amino) ester for efficient delivery of genes and chemotherapeutic agents targeting hepatoma. Polym. Chem. 7, 6340–6353 (2016).
Zhu, H. et al. Multilayered semiconducting polymer nanoparticles with enhanced NIR fluorescence for molecular imaging in cells, zebrafish and mice. Chem. Sci. 7, 5118–5125 (2016).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant number 2017YFA0106100), the National Natural Science Foundation of China (grant numbers 51373017, 51773013, and 51733001), and the Fundamental Research Funds for the Central Universities (Project No. BHYC1705A). Animal studies were approved by the Ethical Committee of Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College and performed under legal protocols.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, L., Zhou, X., Dai, X. et al. Self-destructible polysaccharide nanocomposites with unlockable Au nanorods for high-performance photothermal therapy. NPG Asia Mater 10, 509–521 (2018). https://doi.org/10.1038/s41427-018-0053-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41427-018-0053-2
This article is cited by
-
Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation
Journal of Nanobiotechnology (2022)
-
Polysaccharides-based nanohybrids: Promising candidates for biomedical materials
Science China Materials (2019)