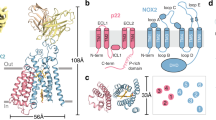
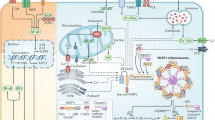
Abstract
Reactive oxygen species (ROS) are pervasive signaling molecules in biological systems. In humans, a lack of ROS causes chronic and extreme bacterial infections, while uncontrolled release of these factors causes pathologies due to excessive inflammation. Professional phagocytes such as neutrophils (PMNs), eosinophils, monocytes, and macrophages use superoxide-generating NADPH oxidase (NOX) as part of their arsenal of antimicrobial mechanisms to produce high levels of ROS. NOX is a multisubunit enzyme complex composed of five essential subunits, two of which are localized in the membrane, while three are localized in the cytosol. In resting phagocytes, the oxidase complex is unassembled and inactive; however, it becomes activated after cytosolic components translocate to the membrane and are assembled into a functional oxidase. The NOX isoforms play a variety of roles in cellular differentiation, development, proliferation, apoptosis, cytoskeletal control, migration, and contraction. Recent studies have identified NOX as a major contributor to disease pathologies, resulting in a shift in focus on inhibiting the formation of potentially harmful free radicals. Therefore, a better understanding of the molecular mechanisms and the transduction pathways involved in NOX-mediated signaling is essential for the development of new therapeutic agents that minimize the hyperproduction of ROS. The current review provides a thorough overview of the various NOX enzymes and their roles in disease pathophysiology, highlights pharmacological strategies, and discusses the importance of computational modeling for future NOX-related studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–67.
Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30:11–26.
Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–41.
Maraldi T. Natural compounds as modulators of NADPH oxidases. Oxid Med Cell Longev. 2013;2013:271602.
Barua S, Kim JY, Yenari MA, Lee JE. The role of NOX inhibitors in neurodegenerative diseases. IBRO Rep. 2019;7:59–69.
Jarman ER, Khambata VS, Cope C, Jones P, Roger J, Ye LY, et al. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am J Respir Cell Mol Biol. 2014;50:158–69.
Cui Y, Wang Y, Li G, Ma W, Zhou XS, Wang J, et al. The Nox1/Nox4 inhibitor attenuates acute lung injury induced by ischemia-reperfusion in mice. PLoS One. 2018;13:e0209444.
Santillo M, Colantuoni A, Mondola P, Guida B, Damiano S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front Physiol. 2015;6:194.
Rastogi R, Geng X, Li F, Ding Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci. 2016;10:301.
Schroder K, Weissmann N, Brandes RP. Organizers and activators: Cytosolic Nox proteins impacting on vascular function. Free Radic Biol Med. 2017;109:22–32.
Giardino G, Cicalese MP, Delmonte O, Migliavacca M, Palterer B, Loffredo L, et al. NADPH oxidase deficiency: a multisystem approach. Oxid Med Cell Longev. 2017;2017:4590127.
El-Benna J, Dang PM-C, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox the phagocyte NADPH oxidase/NOX2 organizer: structure phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–25. https://doi.org/10.3858/emm.2009.41.4.058
Ueno N, Takeya R, Miyano K, Kikuchi H, Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22-dependent manner. J Biol Chem. 2005;280:23328–39. https://doi.org/10.1074/jbc.M414548200
Henríquez-Olguín C, Boronat S, Cabello-Verrugio C, Jaimovich E, Hidalgo E, Jensen TE. The emerging roles of nicotinamide adenine dinucleotide phosphate oxidase 2 in skeletal muscle redox signaling and metabolism. Antioxid Redox Signal. 2019;31:1371–4. https://doi.org/10.1089/ars.2018.7678
Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23.
Gimenez M, Schickling BM, Lopes LR, Miller FJ Jr. Nox1 in cardiovascular diseases: regulation and pathophysiology. Clin Sci. 2016;130:151–65.
Parascandolo A, Laukkanen MO. Carcinogenesis and reactive oxygen species signaling: interaction of the NADPH oxidase NOX1-5 and superoxide dismutase 1-3 signal transduction pathways. Antioxid Redox Signal. 2019;30:443–86.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Magnani F, Nenci S, Millana Fananas E, Ceccon M, Romero E, Fraaije MW, et al. Crystal structures and atomic model of NADPH oxidase. Proc Natl Acad Sci. 2017;114:6764–9.
van der Vliet A, Danyal K, Heppner DE. Dual oxidase: a novel therapeutic target in allergic disease. Br J Pharm. 2018;175:1401–18.
Rolas L, Boussif A, Weiss E, Lettéron P, Haddad O, El-Benna J, et al. NADPH oxidase depletion in neutrophils from patients with cirrhosis and restoration via toll-like receptor 7/8 activation. Gut. 2018;67:1505–16.
Yue L, Wang W, Wang Y, Du T, Shen W, Tang H, et al. Bletilla striata polysaccharide inhibits angiotensin II-induced ROS and inflammation via NOX4 and TLR2 pathways. Int J Biol Macromol. 2016;89:376–88.
Carnevale R, Pastori D, Nocella C, Cammisotto V, Bartimoccia S, Novo M, et al. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Nox2 activation in patients with impaired fasting glucose tolerance: effect of extra-virgin olive oil. Eur J Nutr. 2019;58:843–51.
Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34.
Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, Bhatta R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol. 2018;37:20–36.
El-Zayat SR, Sibaii H, Mannaa FA. Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl Res Cent. 2019;43:187.
Wang Y, Song E, Bai B, Vanhoutte PM. Toll-like receptors mediating vascular malfunction: Lessons from receptor subtypes. Pharm Ther. 2016;158:91–100.
Singh A, Koduru B, Carlisle C, Akhter H, Liu R-M, Schroder K, et al. NADPH oxidase 4 modulates hepatic responses to lipopolysaccharide mediated by Toll-like receptor-4. Sci Rep. 2017;7:14346.
Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–14.
Lorne E, Zmijewski JW, Zhao X, Liu G, Tsuruta Y, Park YJ, et al. Role of extracellular superoxide in neutrophil activation: interactions between xanthine oxidase and TLR4 induce proinflammatory cytokine production. Am J Physiol Cell Physiol. 2008;294:C985–93.
Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017;7:373.
Al-Khafaji AB, Tohme S, Yazdani HO, Miller D, Huang H, Tsung A. Superoxide induces neutrophil extracellular trap formation in a TLR-4 and NOX-dependent mechanism. Mol Med. 2016;22:621–31.
Platnich JM, Muruve DA. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 2019;670:4–14.
Sharma D, Kanneganti TD. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213:617–29.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
Zhen Y, Zhang H. NLRP3 inflammasome and inflammatory bowel disease. Front Immunol. 2019;10:276.
Fangxiao M, Yifan K, Jihong Z, Yan S, Yingchao L. Effect of tripterygium wilfordii polycoride on the NOXs-ROS-NLRP3 inflammasome signaling pathway in mice with ulcerative colitis. Evid Based Complement Altern Med. 2019;2019:9306283.
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Disco. 2018;17:588–606.
Kasper L, König A, Koenig PA, Gresnigt MS, Westman J, Drummond RA, et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9:4260.
Rogiers O, Frising UC, Kucharíková S, Jabra-Rizk MA, van Loo G, Van Dijck P, et al. Candidalysin crucially contributes to nlrp3 inflammasome activation by Candida albicans hyphae. mBio. 2019;10:e02221–18.
Mathur A, Feng S, Hayward JA, Ngo C, Fox D, Atmosukarto II, et al. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat Microbiol. 2019;4:362–74.
You Y, Huang Y, Wang D, Li Y, Wang G, Jin S, et al. Angiotensin (1-7) inhibits arecoline-induced migration and collagen synthesis in human oral myofibroblasts via inhibiting NLRP3 inflammasome activation. J Cell Physiol. 2019;234:4668–80.
Chen Y, Ding SX, Zhang H, Sun ZH, Shen XY, Sun LL, et al. Protective effects of ginsenoside Rg1 on neuronal senescence due to inhibition of NOX2 and NLRP1 inflammasome activation in SAMP8 mice. J Funct Foods. 2020;65:103713.
Wang X, Chu G, Yang Z, Sun Y, Zhou H, Li M. et al. Ethanol directly induced HMGB1 release through NOX2/NLRP1 inflammasome in neuronal cells. Toxicology. 2015;334:104–10.
Sun D, Gao G, Zhong B, Zhang H, Ding S, Sun Z, et al. NLRP1 inflammasome involves in learning and memory impairments and neuronal damages during aging process in mice. Behav Brain Funct. 2021;17:11.
Xu T, Sun L, Shen X, Chen Y, Yin Y, Zhang J, et al. NADPH oxidase 2-mediated NLRP1 inflammasome activation involves in neuronal senescence in hippocampal neurons in vitro. Int Immunopharmacol. 2019;69:60–70.
Sun L, Chen Y, Shen X, Xu T, Yin Y, Zhang H, et al. Inhibition of NOX2-NLRP1 signaling pathway protects against chronic glucocorticoids exposure-induced hippocampal neuronal damage. Int Immunopharmacol. 2019;74:105721.
Cheng L, Chen L, Wei X, Wang Y, Ren Z, Zeng S, et al. NOD2 promotes dopaminergic degeneration regulated by NADPH oxidase 2 in 6-hydroxydopamine model of Parkinson’s disease. J Neuroinflammation. 2018;15:243.
Kong LJ, Liu XQ, Xue Y, Gao W, Lv QZ. Muramyl dipeptide induces reactive oxygen species generation through the NOD2/COX-2/NOX4 signaling pathway in human umbilical vein endothelial cells. J Cardiovasc Pharm. 2018;71:352–8.
Lipinski S, Petersen BS, Barann M, Piecyk A, Tran F, Mayr G, et al. Missense variants in NOX1 and p22phox in a case of very-early-onset inflammatory bowel disease are functionally linked to NOD2. Cold Spring Harb Mol Case Stud. 2019;5:a002428.
Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–80.
Hou L, Zhang L, Hong JS, Zhang D, Zhao J, Wang Q. Nicotinamide adenine dinucleotide phosphate oxidase and neurodegenerative diseases: mechanisms and therapy. Antioxid Redox Signal. 2020;33:374–93.
Esteras N, Kundel F, Amodeo GF, Pavlov EV, Klenerman D, Abramov AY. Insoluble tau aggregates induce neuronal death through modification of membrane ion conductance, activation of voltage-gated calcium channels and NADPH oxidase. Febs J. 2021;288:127–41.
Russo R, Cattaneo F, Lippiello P, Cristiano C, Zurlo F, Castaldo M, et al. Motor coordination and synaptic plasticity deficits are associated with increased cerebellar activity of NADPH oxidase, CAMKII, and PKC at preplaque stage in the TgCRND8 mouse model of Alzheimer’s disease. Neurobiol Aging. 2018;68:123–33.
Rabie MA, Abd El Fattah MA, Nassar NN, El-Abhar HS, Abdallah DM. Angiotensin 1-7 ameliorates 6-hydroxydopamine lesions in hemiparkinsonian rats through activation of MAS receptor/PI3K/Akt/BDNF pathway and inhibition of angiotensin II type-1 receptor/NF-kappaB axis. Biochem Pharm. 2018;151:126–34.
Wu G, Li L, Li HM, Zeng Y, Wu WC. Electroacupuncture ameliorates spatial learning and memory impairment via attenuating NOX2-related oxidative stress in a rat model of Alzheimer’s disease induced by Abeta1-42. Cell Mol Biol. 2017;63:38–45.
Sorce S, Stocker R, Seredenina T, Holmdahl R, Aguzzi A, Chio A, et al. NADPH oxidases as drug targets and biomarkers in neurodegenerative diseases: What is the evidence? Free Radic Biol Med. 2017;112:387–96.
Hemmati-Dinarvand M, Taher-Aghdam AA, Mota A, Zununi Vahed S, Samadi N. Dysregulation of serum NADPH oxidase1 and ferritin levels provides insights into diagnosis of Parkinson’s disease. Clin Biochem. 2017;50:1087–92.
Jiang T, Sun Q, Chen S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol. 2016;147:1–19.
Zhang C, Hou L, Yang J, Che Y, Sun F, Li H, et al. 2,5-Hexanedione induces dopaminergic neurodegeneration through integrin α(M)β2/NADPH oxidase axis-mediated microglial activation. Cell Death Dis. 2018;9:60.
Chung YC, Baek JY, Kim SR, Ko HW, Bok E, Shin WH, et al. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson’s disease. Exp Mol Med. 2017;49:e298.
González-Reyes RE, Nava-Mesa MO, Vargas-Sánchez K, Ariza-Salamanca D, Mora-Muñoz L. Involvement of astrocytes in alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front Mol Neurosci. 2017;10:427.
Chay KO, Nam Koong KY, Hwang S, Kim JK, Bae CS. NADPH oxidase mediates β-amyloid peptide-induced neuronal death in mouse cortical cultures. Chonnam Med J. 2017;53:196–202.
Wyssenbach A, Quintela T, Llavero F, Zugaza JL, Matute C, Alberdi E. Amyloid β-induced astrogliosis is mediated by β1-integrin via NADPH oxidase 2 in Alzheimer’s disease. Aging Cell. 2016;15:1140–52.
Joseph E, Villalobos-Acosta D, Torres-Ramos MA, Farfán-García ED, Gómez-López M, Miliar-García Á, et al. Neuroprotective effects of apocynin and galantamine during the chronic administration of scopolamine in an alzheimer’s disease model. J Mol Neurosci. 2020;70:180–93.
Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science. 2019;365:eaav9518.
Sharma N, Kapoor M, Nehru B. Apocyanin, NADPH oxidase inhibitor prevents lipopolysaccharide induced α-synuclein aggregation and ameliorates motor function deficits in rats: Possible role of biochemical and inflammatory alterations. Behav Brain Res. 2016;296:177–90.
Gallardo-Fernández M, Hornedo-Ortega R, Alonso-Bellido IM, Rodríguez-Gómez JA, Troncoso AM, García-Parrilla MC, et al. Hydroxytyrosol decreases LPS- and α-synuclein-induced microglial activation in vitro. Antioxidants. 2019;9:36.
Hou L, Bao X, Zang C, Yang H, Sun F, Che Y, et al. Integrin CD11b mediates alpha-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018;14:600–8.
Che Y, Hou L, Sun F, Zhang C, Liu X, Piao F, et al. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018;9:435.
Jiang T, Hoekstra J, Heng X, Kang W, Ding J, Liu J, et al. P2X7 receptor is critical in α-synuclein-mediated microglial NADPH oxidase activation. Neurobiol Aging. 2015;36:2304–18.
Rodriguez-Perez AI, Sucunza D, Pedrosa MA, Garrido-Gil P, Kulisevsky J, Lanciego JL, et al. Angiotensin type 1 receptor antagonists protect against alpha-synuclein-induced neuroinflammation and dopaminergic neuron death. Neurotherapeutics. 2018;15:1063–81.
Hou L, Sun F, Sun W, Zhang L, Wang Q. Lesion of the locus coeruleus damages learning and memory performance in paraquat and maneb-induced mouse parkinson’s disease model. Neuroscience. 2019;419:129–40.
Hou L, Che Y, Sun F, Wang Q. Taurine protects noradrenergic locus coeruleus neurons in a mouse Parkinson’s disease model by inhibiting microglial M1 polarization. Amino Acids. 2018;50:547–56.
Hou L, Zhou X, Zhang C, Wang K, Liu X, Che Y, et al. NADPH oxidase-derived H(2)O(2) mediates the regulatory effects of microglia on astrogliosis in experimental models of Parkinson’s disease. Redox Biol. 2017;12:162–70.
Dang DK, Shin EJ, Kim DJ, Tran HQ, Jeong JH, Jang CG, et al. PKCdelta-dependent p47phox activation mediates methamphetamine-induced dopaminergic neurotoxicity. Free Radic Biol Med. 2018;115:318–37.
Morrice JR, Gregory-Evans CY, Shaw CA. Animal models of amyotrophic lateral sclerosis: A comparison of model validity. Neural Regeneration Res. 2018;13:2050–4.
Apolloni S, Fabbrizio P, Parisi C, Amadio S, Volonté C. Clemastine confers neuroprotection and induces an anti-inflammatory phenotype in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Mol Neurobiol. 2016;53:518–31.
Wang T, Cheng J, Wang S, Wang X, Jiang H, Yang Y, et al. α-Lipoic acid attenuates oxidative stress and neurotoxicity via the ERK/Akt-dependent pathway in the mutant hSOD1 related Drosophila model and the NSC34 cell line of amyotrophic lateral sclerosis. Brain Res Bull. 2018;140:299–310.
Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–70.
Seredenina T, Nayernia Z, Sorce S, Maghzal GJ, Filippova A, Ling SC, et al. Evaluation of NADPH oxidases as drug targets in a mouse model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2016;97:95–108.
Kato K, Hecker L. NADPH oxidases: Pathophysiology and therapeutic potential in age-associated pulmonary fibrosis. Redox. Biology 2020;33:101541.
Kurundkar A, Thannickal VJ. Redox mechanisms in age-related lung fibrosis. Redox Biol. 2016;9:67–76.
Liu B, Rong Y, Sun D, Li W, Chen H, Cao B, et al. Costunolide inhibits pulmonary fibrosis via regulating NF-kB and TGF-beta1/Smad2/Nrf2-NOX4 signaling pathways. Biochem Biophys Res Commun. 2019;510:329–33.
Wu YC, Wang WT, Lee SS, Kuo YR, Wang YC, Yen SJ, et al. Glucagon-like peptide-1 receptor agonist attenuates autophagy to ameliorate pulmonary arterial hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3beta pathways. Int J Mol Sci. 2019;20:3435.
Kracun D, Klop M, Knirsch A, Petry A, Kanchev I, Chalupsky K, et al. NADPH oxidases and HIF1 promote cardiac dysfunction and pulmonary hypertension in response to glucocorticoid excess. Redox Biol. 2020;34:101536.
Hollins F, Sutcliffe A, Gomez E, Berair R, Russell R, Szyndralewiez C, et al. Airway smooth muscle NOX4 is upregulated and modulates ROS generation in COPD. Respir Res. 2016;17:84.
Hsu PS, Lin CM, Chang JF, Wu CS, Sia KC, Lee IT, et al. Participation of NADPH oxidase-related reactive oxygen species in leptin-promoted pulmonary inflammation: regulation of cPLA2alpha and COX-2 expression. Int J Mol Sci. 2019;20:1078.
Zhang Y, Shan P, Srivastava A, Jiang G, Zhang X, Lee PJ. An endothelial Hsp70-TLR4 axis limits Nox3 expression and protects against oxidant injury in lungs. Antioxid Redox Signal. 2016;24:991–1012.
Wieczfinska J, Sokolowska M, Pawliczak R. NOX modifiers-just a step away from application in the therapy of airway inflammation? Antioxid Redox Signal. 2015;23:428–45.
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Prim. 2017;3:17074.
Waters DW, Blokland KEC, Pathinayake PS, Burgess JK, Mutsaers SE, Prele CM, et al. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315:L162–L72.
Veith C, Boots AW, Idris M, van Schooten F-J, van der Vliet A. Redox imbalance in idiopathic pulmonary fibrosis: a role for oxidant cross-talk between NADPH oxidase enzymes and mitochondria. Antioxid Redox Signal. 2019;31:1092–115. https://doi.org/10.1089/ars.2019.7742
Li L, Cai L, Zheng L, Hu Y, Yuan W, Guo Z, et al. Gefitinib inhibits bleomycin-induced pulmonary fibrosis via alleviating the oxidative damage in mice. Oxid Med Cell Longev. 2018;2018:1–12. https://doi.org/10.1155/2018/8249693
Wang CL, Kang J, Li ZH. [Increased expression of NADPH oxidase p47-PHOX and p67-PHOX factor in idiopathic pulmonary fibrosis]. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30:265–8.
Jarman ER, Khambata VS, Yun Ye L, Cheung K, Thomas M, Duggan N, et al. A translational preclinical model of interstitial pulmonary fibrosis and pulmonary hypertension: mechanistic pathways driving disease pathophysiology. Physiol Rep. 2014;2:e12133.
Liu RM, Desai LP. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015;6:565–77.
Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15:607–19.
Maximillian R, Richter T. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock. 2010;3:43–51. https://doi.org/10.4103/0974-2700.58663
Wu F, Szczepaniak WS, Shiva S, Liu H, Wang Y, Wang L, et al. Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L987–97.
Whitmore LC, Hilkin BM, Goss KL, Wahle EM, Colaizy TT, Boggiatto PM, et al. NOX2 protects against prolonged inflammation, lung injury, and mortality following systemic insults. J Innate Immun. 2013;5:565–80.
Huang W, Xiong YQ, Chen YL, Cheng Y, Wang RR. NOX2 is involved in CB2-mediated protection against lung ischemia-reperfusion injury in mice. Int J Clin Exp Pathol. 2020;13:277–85.
Stanton RC. Glucose-6-phosphate dehydrogenase NADPH and cell survival. IUBMB Life. 2012;64:362–9. https://doi.org/10.1002/iub.1017
Nadeem A, Al-Harbi NO, Ahmad SF, Ibrahim KE, Siddiqui N, Al-Harbi MM. Glucose-6-phosphate dehydrogenase inhibition attenuates acute lung injury through reduction in NADPH oxidase-derived reactive oxygen species. Clin Exp Immunol. 2018;191:279–87.
Chen W, Chen YY, Tsai CF, Chen SC, Lin MS, Ware LB, et al. Incidence and outcomes of acute respiratory distress syndrome: a nationwide registry-based study in Taiwan, 1997 to 2011. Medicine. 2015;94:e1849.
Jensen JS, Itenov TS, Thormar KM, Hein L, Mohr TT, Andersen MH, et al. Prediction of non-recovery from ventilator-demanding acute respiratory failure, ARDS and death using lung damage biomarkers: data from a 1200-patient critical care randomized trial. Ann Intensive Care. 2016;6:114.
Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, Black SM. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv Exp Med Biol. 2017;967:105–37.
Wells JM, Iyer AS, Rahaghi FN, Bhatt SP, Gupta H, Denney TS, et al. Pulmonary artery enlargement is associated with right ventricular dysfunction and loss of blood volume in small pulmonary vessels in chronic obstructive pulmonary disease. Circ Cardiovasc Imaging. 2015;8:e002546.
Boukhenouna S, Wilson MA, Bahmed K, Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2018;2018:1–9. https://doi.org/10.1155/2018/5730395
Guo X, Fan Y, Cui J, Hao B, Zhu L, Sun X, et al. NOX4 expression and distal arteriolar remodeling correlate with pulmonary hypertension in COPD. BMC Pulm Med. 2018;18:111.
Liu X, Hao B, Ma A, He J, Liu X, Chen J. The expression of NOX4 in smooth muscles of small airway correlates with the disease severity of COPD. Biomed Res Int. 2016;2016:2891810.
Seimetz M, Sommer N, Bednorz M, Pak O, Veith C, Hadzic S, et al. NADPH oxidase subunit NOXO1 is a target for emphysema treatment in COPD. Nat Metab. 2020;2:532–46.
Li H, Han X, Hu Z, Huang J, Chen J, Hixson JE, et al. Associations of NADPH oxidase-related genes with blood pressure changes and incident hypertension: The GenSalt Study. J Hum Hypertens. 2018;32:287–93.
Lu W, Kang J, Hu K, Tang S, Zhou X, Xu L, et al. The role of the Nox4-derived ROS-mediated RhoA/Rho kinase pathway in rat hypertension induced by chronic intermittent hypoxia. Sleep Breath. 2017;21:667–77.
Song Q, Zhang Y, Han X, Zhang X, Gao Y, Zhang J, et al. Potential mechanisms underlying the protective effects of salvianic acid A against atherosclerosis in vivo and vitro. Biomed Pharmacother. 2019;109:945–56.
Lu Z, Wang F, Yu P, Wang X, Wang Y, Tang ST, et al. Inhibition of miR-29b suppresses MAPK signaling pathway through targeting SPRY1 in atherosclerosis. Vasc Pharm. 2018;102:29–36.
Simplicio JA, do Vale GT, Gonzaga NA, Leite LN, Hipolito UV, Pereira CA, et al. Reactive oxygen species derived from NAD(P)H oxidase play a role on ethanol-induced hypertension and endothelial dysfunction in rat resistance arteries. J Physiol Biochem. 2017;73:5–16.
Shin YK, Han AY, Hsieh YS, Kwon S, Kim J, Lee KW, et al. Lancemaside A from Codonopsis lanceolata prevents hypertension by inhibiting NADPH oxidase 2-mediated MAPK signalling and improving NO bioavailability in rats. J Pharm Pharm. 2019;71:1458–68.
Zhang Y, Murugesan P, Huang K, Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol. 2020;17:170–94.
Knock GA. NADPH oxidase in the vasculature: Expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic Biol Med. 2019;145:385–427.
Li Y, Pagano PJ. Microvascular NADPH oxidase in health and disease. Free Radic Biol Med. 2017;109:33–47.
Li WJ, Liu Y, Wang JJ, Zhang YL, Lai S, Xia YL, et al. “Angiotensin II memory” contributes to the development of hypertension and vascular injury via activation of NADPH oxidase. Life Sci. 2016;149:18–24.
Liang YF, Zhang DD, Yu XJ, Gao HL, Liu KL, Qi J, et al. Hydrogen sulfide in paraventricular nucleus attenuates blood pressure by regulating oxidative stress and inflammatory cytokines in high salt-induced hypertension. Toxicol Lett. 2017;270:62–71.
Marchi KC, Ceron CS, Muniz JJ, De Martinis BS, Tanus-Santos JE, Tirapelli CR. NADPH oxidase plays a role on ethanol-induced hypertension and reactive oxygen species generation in the vasculature. Alcohol Alcohol. 2016;51:522–34.
Huang YP, Jin HY, Yu HP. Inhibitory effects of alpha-lipoic acid on oxidative stress in the rostral ventrolateral medulla in rats with salt-induced hypertension. Int J Mol Med. 2017;39:430–6.
Qi J, Yu XJ, Shi XL, Gao HL, Yi QY, Tan H, et al. NF-kappaB blockade in hypothalamic paraventricular nucleus inhibits high-salt-induced hypertension through NLRP3 and caspase-1. Cardiovasc Toxicol. 2016;16:345–54.
Niazi ZR, Silva GC, Ribeiro TP, León-González AJ, Kassem M, Mirajkar A, et al. EPA:DHA 6:1 prevents angiotensin II-induced hypertension and endothelial dysfunction in rats: role of NADPH oxidase- and COX-derived oxidative stress. Hypertens Res. 2017;40:966–75.
Ma MM, Gao M, Guo KM, Wang M, Li XY, Zeng XL, et al. TMEM16A contributes to endothelial dysfunction by facilitating Nox2 NADPH oxidase-derived reactive oxygen species generation in hypertension. Hypertension. 2017;69:892–901.
Helfinger V, Palfi K, Weigert A, Schröder K. The NADPH oxidase nox4 controls macrophage polarization in an NFκB-dependent manner. Oxid Med Cell Longev. 2019;2019:3264858.
Li X, Lin Y, Zhou H, Li Y, Wang A, Wang H, et al. Puerarin protects against endothelial dysfunction and end-organ damage in Ang II-induced hypertension. Clin Exp Hypertens. 2017;39:58–64.
Liang GZ, Cheng LM, Chen XF, Li YJ, Li XL, Guan YY, et al. ClC-3 promotes angiotensin II-induced reactive oxygen species production in endothelial cells by facilitating Nox2 NADPH oxidase complex formation. Acta Pharm Sin. 2018;39:1725–34.
Ling WC, Mustafa MR, Vanhoutte PM, Murugan DD. Chronic administration of sodium nitrite prevents hypertension and protects arterial endothelial function by reducing oxidative stress in angiotensin II-infused mice. Vasc Pharm. 2018;102:11–20.
Kang Y, Ding L, Dai H, Wang F, Zhou H, Gao Q, et al. Intermedin in paraventricular nucleus attenuates ang II-induced sympathoexcitation through the inhibition of NADPH oxidase-dependent ROS generation in obese rats with hypertension. Int J Mol Sci. 2019;20:4217.
Radwan E, Mali V, Haddox S, El-Noweihi A, Mandour M, Ren J, et al. Treg cells depletion is a mechanism that drives microvascular dysfunction in mice with established hypertension. Biochim Biophys Acta Mol Basis Dis. 2019;1865:403–12.
Camargo LL, Harvey AP, Rios FJ, Tsiropoulou S, Da Silva RNO, Cao Z, et al. Vascular nox (NADPH Oxidase) compartmentalization, protein hyperoxidation, and endoplasmic reticulum stress response in hypertension. Hypertension. 2018;72:235–46.
Sciarretta S, Yee D, Ammann P, Nagarajan N, Volpe M, Frati G, et al. Role of NADPH oxidase in the regulation of autophagy in cardiomyocytes. Clin Sci. 2014;128:387–403.
Mei Y, Thompson MD, Cohen RA, Tong X. Autophagy and oxidative stress in cardiovascular diseases. Biochim Biophys Acta. 2015;1852:243–51.
Mo J, Yang R, Li F, Zhang X, He B, Zhang Y, et al. Scutellarin protects against vascular endothelial dysfunction and prevents atherosclerosis via antioxidation. Phytomedicine. 2018;42:66–74.
Kim J, Seo M, Kim SK, Bae YS. Flagellin-induced NADPH oxidase 4 activation is involved in atherosclerosis. Sci Rep. 2016;6:25437.
Sfyri P, Matsakas A. Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: emphasis on apolipoprotein E deficiency and peripheral arterial disease. J Biomed Sci. 2017;24:42.
Liang W, Wang Q, Ma H, Yan W, Yang J. Knockout of low molecular weight FGF2 attenuates atherosclerosis by reducing macrophage infiltration and oxidative stress in mice. Cell Physiol Biochem. 2018;45:1434–43.
Sfyri PP, Yuldasheva NY, Tzimou A, Giallourou N, Crispi V, Aburima A, et al. Attenuation of oxidative stress-induced lesions in skeletal muscle in a mouse model of obesity-independent hyperlipidaemia and atherosclerosis through the inhibition of Nox2 activity. Free Radic Biol Med. 2018;129:504–19.
Peng Y, Xu J, Zeng Y, Chen L, Xu XL. Polydatin attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of reverse cholesterol transport. Phytomedicine. 2019;62:152935.
Shu Q, Lai S, Wang XM, Zhang YL, Yang XL, Bi HL, et al. Administration of ubiquitin-activating enzyme UBA1 inhibitor PYR-41 attenuates angiotensin II-induced cardiac remodeling in mice. Biochem Biophys Res Commun. 2018;505:317–24.
Charbonneau M-E, Passalacqua KD, Hagen SE, Showalter HD, Wobus CE, O’Riordan MXD. Perturbation of ubiquitin homeostasis promotes macrophage oxidative defenses. Sci Rep. 2019;9:10245.
Liao J, Yang X, Lin Q, Liu S, Xie Y, Xia Y, et al. Inhibition of the ubiquitin-activating enzyme UBA1 suppresses diet-induced atherosclerosis in apolipoprotein E-knockout mice. J Immunol Res. 2020;2020:7812709.
Manea SA, Vlad ML, Fenyo IM, Lazar AG, Raicu M, Muresian H, et al. Pharmacological inhibition of histone deacetylase reduces NADPH oxidase expression, oxidative stress and the progression of atherosclerotic lesions in hypercholesterolemic apolipoprotein E-deficient mice; potential implications for human atherosclerosis. Redox Biol. 2020;28:101338.
Vlad ML, Manea SA, Lazar AG, Raicu M, Muresian H, Simionescu M, et al. Histone acetyltransferase-dependent pathways mediate upregulation of NADPH oxidase 5 in human macrophages under inflammatory conditions: a potential mechanism of reactive oxygen species overproduction in atherosclerosis. Oxid Med Cell Longev. 2019;2019:3201062.
Cheng Y, Zhou M, Zhou W. MicroRNA-30e regulates TGF-β-mediated NADPH oxidase 4-dependent oxidative stress by Snai1 in atherosclerosis. Int J Mol Med. 2019;43:1806–16.
Pejenaute Á, Cortés A, Marqués J, Montero L, Beloqui Ó, Fortuño A, et al. NADPH oxidase overactivity underlies telomere shortening in human atherosclerosis. Int J Mol Sci. 2020;21:1434.
Shi Q, Viswanadhapalli S, Friedrichs WE, Velagapudi C, Szyndralewiez C, Bansal S, et al. Nox4 is a target for tuberin deficiency syndrome. Sci Rep. 2018;8:3781.
Jeong BY, Park SR, Cho S, Yu SL, Lee HY, Park CG, et al. TGF-beta-mediated NADPH oxidase 4-dependent oxidative stress promotes colistin-induced acute kidney injury. J Antimicrob Chemother. 2018;73:962–72.
Jeong BY, Lee HY, Park CG, Kang J, Yu SL, Choi DR, et al. Oxidative stress caused by activation of NADPH oxidase 4 promotes contrast-induced acute kidney injury. PLoS One. 2018;13:e0191034.
Meng XM, Ren GL, Gao L, Yang Q, Li HD, Wu WF, et al. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab Invest. 2018;98:63–78.
Cha JJ, Min HS, Kim KT, Kim JE, Ghee JY, Kim HW, et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab Invest. 2017;97:419–31.
Sureshbabu A, Patino E, Ma KC, Laursen K, Finkelsztein EJ, Akchurin O, et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight. 2018;3:e98411.
Al-Harbi NO, Nadeem A, Ahmad SF, Alanazi MM, Aldossari AA, Alasmari F. Amelioration of sepsis-induced acute kidney injury through inhibition of inflammatory cytokines and oxidative stress in dendritic cells and neutrophils respectively in mice: Role of spleen tyrosine kinase signaling. Biochimie. 2019;158:102–10.
Sawhney S, Fraser SD. Epidemiology of AKI: utilizing large databases to determine the burden of AKI. Adv Chronic Kidney Dis. 2017;24:194–204.
Cho S, Yu SL, Kang J, Jeong BY, Lee HY, Park CG, et al. NADPH oxidase 4 mediates TGF-β1/Smad signaling pathway induced acute kidney injury in hypoxia. PLoS One. 2019;14:e0219483.
Gyurászová M, Gurecká R, Bábíčková J, Tóthová Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxid Med Cell Longev. 2020;2020:5478708.
Galvan DL, Green NH, Danesh FR. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92:1051–7.
Karanovic D, Grujic-Milanovic J, Miloradovic Z, Ivanov M, Jovovic D, Vajic UJ, et al. Effects of single and combined losartan and tempol treatments on oxidative stress, kidney structure and function in spontaneously hypertensive rats with early course of proteinuric nephropathy. PLoS One. 2016;11:e0161706.
Djamali A, Wilson NA, Sadowski EA, Zha W, Niles D, Hafez O, et al. Nox2 and cyclosporine-induced renal hypoxia. Transplantation. 2016;100:1198–210.
Vodosek Hojs N, Bevc S, Ekart R, Hojs R. Oxidative stress markers in chronic kidney disease with emphasis on diabetic nephropathy. Antioxidants. 2020;9:925.
Kuchta A, Pacanis A, Kortas-Stempak B, Cwiklinska A, Zietkiewicz M, Renke M, et al. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press Res. 2011;34:12–9.
Duni A, Liakopoulos V, Roumeliotis S, Peschos D, Dounousi E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: untangling ariadne’s thread. Int J Mol Sci. 2019;20:3711.
Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, et al. NAD(P)H oxidase mediates TGF-β1–induced activation of kidney myofibroblasts. J Am Soc Nephrol. 2010;21:93–102. https://doi.org/10.1681/ASN.2009020146
Nezu M, Suzuki N, Yamamoto M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am J Nephrol. 2017;45:473–83.
Lee JH, Kim D, Oh YS, Jun HS. Lysophosphatidic acid signaling in diabetic nephropathy. Int J Mol Sci. 2019;20:2850.
Zhang J, Yang S, Li H, Chen F, Shi J. Naringin ameliorates diabetic nephropathy by inhibiting NADPH oxidase 4. Eur J Pharm. 2017;804:1–6.
Lopez-Sanz L, Bernal S, Recio C, Lazaro I, Oguiza A, Melgar A, et al. SOCS1-targeted therapy ameliorates renal and vascular oxidative stress in diabetes via STAT1 and PI3K inhibition. Lab Invest. 2018;98:1276–90.
Cheng YS, Chao J, Chen C, Lv LL, Han YC, Liu BC. The PKCbeta-p66shc-NADPH oxidase pathway plays a crucial role in diabetic nephropathy. J Pharm Pharm. 2019;71:338–47.
Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev. 2017;33:e2841.
Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, et al. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes. 2017;66:2691–703.
Holterman CE, Read NC, Kennedy CR. Nox and renal disease. Clin Sci. 2015;128:465–81.
Stinghen AE, Massy ZA, Vlassara H, Striker GE, Boullier A. Uremic toxicity of advanced glycation end products in CKD. J Am Soc Nephrol. 2016;27:354–70.
Matsui T, Higashimoto Y, Nishino Y, Nakamura N, Fukami K, Yamagishi SI. RAGE-aptamer blocks the development and progression of experimental diabetic nephropathy. Diabetes. 2017;66:1683–95.
Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605.
Yao M, Gao F, Wang X, Shi Y, Liu S, Duan H. Nox4 is involved in high glucose-induced apoptosis in renal tubular epithelial cells via Notch pathway. Mol Med Rep. 2017;15:4319–25.
Cui Y, Shi Y, Bao Y, Wang S, Hua Q, Liu Y. Zingerone attenuates diabetic nephropathy through inhibition of nicotinamide adenine dinucleotide phosphate oxidase 4. Biomed Pharmacother. 2018;99:422–30.
Reis J, Massari M, Marchese S, Ceccon M, Aalbers FS, Corana F, et al. A closer look into NADPH oxidase inhibitors: Validation and insight into their mechanism of action. Redox Biology. 2020;32:101466.
Kovacic H. 2020, A decisive decade for NADPH oxidases inhibitors. Antioxid Redox Signal. 2020;33:329–31.
Chocry M, Leloup L. The NADPH oxidase family and its inhibitors. Antioxid Redox Signal. 2020;33:332–53.
Li Y, Cifuentes-Pagano E, DeVallance ER, de Jesus DS, Sahoo S, Meijles DN, et al. NADPH oxidase 2 inhibitors CPP11G and CPP11H attenuate endothelial cell inflammation & vessel dysfunction and restore mouse hind-limb flow. Redox Biology. 2019;22:101143.
de Jesus DS, DeVallance E, Li Y, Falabella M, Guimaraes D, Shiva S, et al. Nox1/Ref-1-mediated activation of CREB promotes Gremlin1-driven endothelial cell proliferation and migration. Redox Biol. 2019;22:101138.
Levy C. Novel therapies for cholestatic liver disease. Gastroenterol Hepatol. 2019;15:493–6.
Galoosian A, Hanlon C, Zhang J, Holt EW, Yimam KK. Clinical updates in primary biliary cholangitis: trends, epidemiology, diagnostics, and new therapeutic approaches. J Clin Transl Hepatol. 2020;8:49–60.
Petronio MS, Zeraik ML, da Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules. 2013;18:2821–39.
Augsburger F, Filippova A, Rasti D, Seredenina T, Lam M, Maghzal G, et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019;26:101272.
Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, Schmidt HHNOX1. 2, 4, 5: counting out oxidative stress. Br J Pharm. 2011;164:866–83.
ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, et al. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–41.
Zhao J, Sun T, Wu JJ, Cao YF, Fang ZZ, Sun HZ, et al. Inhibition of human CYP3A4 and CYP3A5 enzymes by gomisin C and gomisin G, two lignan analogs derived from Schisandra chinensis. Fitoterapia. 2017;119:26–31.
Yang J, Li J, Wang Q, Xing Y, Tan Z, Kang Q. Novel NADPH oxidase inhibitor VAS2870 suppresses TGFbetadependent epithelialtomesenchymal transition in retinal pigment epithelial cells. Int J Mol Med. 2018;42:123–30.
Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8:e1000479 https://doi.org/10.1371/journal.pbio.1000479
Szilagyi JT, Mishin V, Heck DE, Jan YH, Aleksunes LM, Richardson JR, et al. Selective targeting of heme protein in cytochrome P450 and nitric oxide synthase by diphenyleneiodonium. Toxicol Sci. 2016;151:150–9.
Sun J, Ming L, Shang F, Shen L, Chen J, Jin Y. Apocynin suppression of NADPH oxidase reverses the aging process in mesenchymal stem cells to promote osteogenesis and increase bone mass. Sci Rep. 2015;5:18572.
Lu J, Risbood P, Kane CT Jr, Hossain MT, Anderson L, Hill K, et al. Characterization of potent and selective iodonium-class inhibitors of NADPH oxidases. Biochem Pharm. 2017;143:25–38.
Nagel S, Genius J, Heiland S, Horstmann S, Gardner H, Wagner S. Diphenyleneiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral ischemia of the rat. Brain Res. 2007;1132:210–7.
Byrnes KR, Washington PM, Knoblach SM, Hoffman E, Faden AI. Delayed inflammatory mRNA and protein expression after spinal cord injury. J Neuroinflammation. 2011;8:130.
He YF, Chen HJ, Qian LH, He LF, Buzby JS. Diphenyleneiodonium protects preoligodendrocytes against endotoxin-activated microglial NADPH oxidase-generated peroxynitrite in a neonatal rat model of periventricular leukomalacia. Brain Res. 2013;1492:108–21.
Wang Q, Qian L, Chen SH, Chu CH, Wilson B, Oyarzabal E, et al. Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson’s disease models. Brain. 2015;138:1247–62.
Kim SK, Rho SJ, Kim SH, Kim SY, Song SH, Yoo JY, et al. Protective effects of diphenyleneiodonium, an NADPH oxidase inhibitor, on lipopolysaccharide-induced acute lung injury. Clin Exp Pharm Physiol. 2019;46:153–62.
Lapchak PA, Zivin JA. Ebselen, a seleno-organic antioxidant, is neuroprotective after embolic strokes in rabbits: synergism with low-dose tissue plasminogen activator. Stroke. 2003;34:2013–8.
Cifuentes-Pagano ME, Meijles DN, Pagano PJ. Nox inhibitors & therapies: rational design of peptidic and small molecule inhibitors. Curr Pharm Des. 2015;21:6023–35.
Solbak SMO, Zang J, Narayanan D, Hoj LJ, Bucciarelli S, Softley C, et al. Developing inhibitors of the p47phox-p22phox protein-protein interaction by fragment-based drug discovery. J Med Chem. 2020;63:1156–77.
Unsal C, Oran M, Albayrak Y, Aktas C, Erboga M, Topcu B, et al. Neuroprotective effect of ebselen against intracerebroventricular streptozotocin-induced neuronal apoptosis and oxidative stress in rats. Toxicol Ind Health. 2016;32:730–40.
Jia ZQ, Li SQ, Qiao WQ, Xu WZ, Xing JW, Liu JT, et al. Ebselen protects mitochondrial function and oxidative stress while inhibiting the mitochondrial apoptosis pathway after acute spinal cord injury. Neurosci Lett. 2018;678:110–7.
Park C, Choi SH, Jeong J-W, Han MH, Lee H, Hong SH, et al. Honokiol ameliorates oxidative stress[1]induced DNA damage and apoptosis of c2c12 myoblasts by ROS generation and mitochondrial pathway. Anim Cells Syst (Seoul). 2019;24:60–8. https://doi.org/10.1080/19768354.2019.1706634
Wang M, Li Y, Ni C, Song G. Honokiol attenuates oligomeric amyloid beta1-42-induced alzheimer’s disease in mice through attenuating mitochondrial apoptosis and inhibiting the nuclear factor kappa-B signaling pathway. Cell Physiol Biochem. 2017;43:69–81.
Kim HJ, Yoo HY, Zhang YH, Kim WK, Kim SJ. Biphasic augmentation of alpha-adrenergic contraction by plumbagin in rat systemic arteries. Korean J Physiol Pharm. 2017;21:687–94.
Kim JY, Park J, Lee JE, Yenari MA. NOX inhibitors - a promising avenue for ischemic stroke. Exp Neurobiol. 2017;26:195–205.
Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, et al. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med. 2011;51:1116–25.
Kumar A, Barrett JP, Alvarez-Croda DM, Stoica BA, Faden AI, Loane DJ. NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav Immun. 2016;58:291–309.
Pal R, Bajaj L, Sharma J, Palmieri M, Di Ronza A, Lotfi P, et al. NADPH oxidase promotes Parkinsonian phenotypes by impairing autophagic flux in an mTORC1-independent fashion in a cellular model of Parkinson’s disease. Sci Rep. 2016;6:22866.
Saw S, Kale SL, Arora N. Serine protease inhibitor attenuates ovalbumin induced inflammation in mouse model of allergic airway disease. PLoS One. 2012;7:e41107.
Wang L, Yang T, Wang C. Are statins beneficial for the treatment of pulmonary hypertension? Chronic Dis Transl Med. 2017;3:213–20.
Banfi C, Baetta R, Gianazza E, Tremoli E. Technological advances and proteomic applications in drug discovery and target deconvolution: identification of the pleiotropic effects of statins. Drug Disco Today. 2017;22:848–69.
Chen IC, Tan MS, Wu BN, Chai CY, Yeh JL, Chou SH, et al. Statins ameliorate pulmonary hypertension secondary to left ventricular dysfunction through the Rho-kinase pathway and NADPH oxidase. Pediatr Pulmonol. 2017;52:443–57.
Tong H, Zhang X, Meng X, Lu L, Mai D, Qu S. Simvastatin inhibits activation of NADPH oxidase/p38 MAPK pathway and enhances expression of antioxidant protein in parkinson disease models. Front Mol Neurosci. 2018;11:165.
Soni N, Madhusudhan MS. Computational modeling of protein assemblies. Curr Opin Struct Biol. 2017;44:179–89.
Kaur G, Guruprasad K, Temple BRS, Shirvanyants DG, Dokholyan NV, Pati PK. Structural complexity and functional diversity of plant NADPH oxidases. Amino Acids. 2018;50:79–94.
Meijles DN, Howlin BJ, Li JM. Consensus in silico computational modelling of the p22phox subunit of the NADPH oxidase. Comput Biol Chem. 2012;39:6–13.
Yadav DK, Adhikari M, Kumar S, Ghimire B, Han I, Kim MH, et al. Cold atmospheric plasma generated reactive species aided inhibitory effects on human melanoma cells: an in vitro and in silico study. Sci Rep. 2020;10:3396.
Bosco EE, Kumar S, Marchioni F, Biesiada J, Kordos M, Szczur K, et al. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chem Biol. 2012;19:228–42.
Macias-Perez ME, Martinez-Ramos F, Padilla M, II, Correa-Basurto J, Kispert L, Mendieta-Wejebe JE, et al. Ethers and esters derived from apocynin avoid the interaction between p47phox and p22phox subunits of NADPH oxidase: evaluation in vitro and in silico. Biosci Rep. 2013;33:e00055.
Acknowledgements
This work was supported by the Young Clinical Scientist Award from the Flight Attendant Medical Research Institute (FAMRI- 123253_YCSA_Faculty) and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers 5P20 GM103424-18 and 3 P20 GM103424-15S1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Begum, R., Thota, S., Abdulkadir, A. et al. NADPH oxidase family proteins: signaling dynamics to disease management. Cell Mol Immunol 19, 660–686 (2022). https://doi.org/10.1038/s41423-022-00858-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-022-00858-1
Keywords
This article is cited by
-
Role of reactive oxygen species in myelodysplastic syndromes
Cellular & Molecular Biology Letters (2024)
-
Mycobacterium tuberculosis inhibits METTL14-mediated m6A methylation of Nox2 mRNA and suppresses anti-TB immunity
Cell Discovery (2024)
-
Role and anti-inflammatory mechanisms of acupuncture and moxibustion therapy on pain relief through NOX-ROS-NLRP3 pathway in CCI rats models
Molecular Biology Reports (2023)
-
Interplay between proteasome function and inflammatory responses in e-cig vapor condensate-challenged lung epithelial cells
Archives of Toxicology (2023)