Abstract
The innate immune system plays a crucial role in the host defense against viral and microbial infection. Exosomes constitute a subset of extracellular vesicles (EVs) that can be released by almost all cell types. Owing to their capacity to shield the payload from degradation and to evade recognition and subsequent removal by the immune system, exosomes efficiently transport functional components to recipient cells. Accumulating evidence has recently shown that exosomes derived from tumor cells, host cells and even bacteria and parasites mediate the communication between the invader and innate immune cells and thus play an irreplaceable function in the dissemination of pathogens and donor cell-derived molecules, modulating the innate immune responses of the host. In this review, we describe the current understanding of EVs (mainly focusing on exosomes) and summarize and discuss their crucial roles in determining innate immune responses. Additionally, we discuss the potential of using exosomes as biomarkers and cancer vaccines in diagnostic and therapeutic applications.
Similar content being viewed by others
Introduction
It is becoming increasingly evident that almost all living cells can secrete extracellular vesicles (EVs), including microvesicles (MVs), which are also known as microparticles and ectosomes, exosomes and apoptotic bodies.1,2,3 The size of an exosome ranges between 30 and 150 nm in diameter, whereas that of a microvesicle (0.1–2 μm) and an apoptotic body (1–5 μm) is usually larger.4,5,6 Recently, two novel subpopulations of exosomes (large exosome vesicles, Exo-L, 90–120 nm; small exosome vesicles, Exo-S, 60–80 nm) and an abundant population of nonmembranous nanoparticles termed ‘exomeres’ (~35 nm) were identified.7 As a novel mediator of intercellular communication, EVs carry bioactive molecules such as proteins, lipids, multiple RNA species (microRNAs, mRNAs, and long non-coding RNAs), and even DNA fragments from donor to recipient cells.8,9,10,11,12,13,14,15 The largely selective content packaged into EVs mostly reflects the aims and functions of the parent cells, and EVs are found in a number of human body fluids, such as blood plasma, urine, saliva, sputum, and breast milk.16,17,18,19,20 In addition to eukaryotic cell types, EVs can also be secreted by plant cells and pathogens, including bacteria, archaea, and fungi, suggesting a highly evolutionarily conserved function as a mode of intercellular communication.
Exosomes were first identified in 1981, originally termed shedding vesicles, because of their 5′-nucleotidase activity, which is derived from various normal and neoplastic cell lines.21 Exosomes not only originate from cells in the endosomal pathway via the formation of multivesicular bodies (MVBs) but also bud from the plasma membrane,3,22,23 whereas microvesicles are secreted only by shedding or outward budding of the plasma membrane.24,25 Exosomal budding of the plasma membrane has been observed and is supported by multiple experiments such as electron microscopy and atomic force microscopy, strongly supporting the argument against the widely accepted endosome-only model of exosome biogenesis.22,23,26,27 This prevailing thought may be the result of observational bias caused by the use of electron microscopy, in which intact cells with MVBs are easily recognized, but exosomes budding from the plasma membrane may be undersampled. Based on their different cellular origins, exosomes play distinct roles in normal physiological processes, such as the immune response, cell proliferation, inflammation, metabolism and neuronal function, and in different stages of diseases, including cancer.1,5,28,29,30,31 Moreover, other vesicles, such as oncosomes and melanosomes, also play roles in immune control and in cancer. Oncosomes are atypically large (1–10 µm diameter) cancer-derived EVs originating from shedding membrane blebs.32 They contain abundant molecules and are associated with advanced disease.33,34,35 For example, oncosomes containing Cav-1, a serum biomarker of metastatic prostate cancer, have been correlated with, and can serve to distinguish, patients with metastatic disease.36,37 Melanosomal microvesicles, also called melanosomes, are specialized organelles in melanocytic cells and are devoted to melanin pigment synthesis and storage.38,39 It was suggested that the release of FasL-bearing melanosomal microvesicles mediates the apoptosis of T lymphocytes at the tumor site, representing a possible mechanism by which tumor cells can eradicate antitumor T-cell reactivity.40 In addition, exosomes are also being implicated as diagnostic biomarkers for diseases because they have high stability, reach sufficient concentrations in the circulation and contain a variety of content, such as proteins and RNAs, that reflect their parental cell.20,41
In recent years, studies about exosomes, immunity and their interplay in human diseases have received increasing attention. The innate immune system is composed of a network of cells, including monocytes/macrophages, dendritic cells (DCs), neutrophils and natural killers (NK) cells, that mediate the earliest interactions between host and pathogens.42 Innate immunity is the first line of defense against any invading substances, including viral infections, and plays a key role in the elimination of viruses from a host. Pattern-recognition receptors (PRRs) that recognize viral nucleic acids include Toll-like receptors (TLRs), RIG-I-like receptors (RLRs) and certain DNA sensors, such as cGAS.43,44,45,46,47 PRRs recognize various pathogen-associated molecular patterns (PAMPs), including DNA and RNA from bacteria and viruses and danger-associated molecular patterns (DAMPs).48,49 Different PRRs respond to diverse PAMPs, activate specific signaling pathways, trigger the expression of antiviral type I interferons (IFNs) and pro-inflammatory cytokines, and lead to distinct antipathogen responses.50,51,52,53 In this review, we impart basic knowledge of EVs and exosomes and describe the latest important findings on the mechanisms underlying EV and exosome involvement in innate immune modulation. We also summarize their therapeutic potential.
Biological characteristics of exosomes
Exosome biogenesis
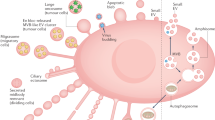
In addition to direct outward budding from the plasma membrane, exosomes can also originate from intraluminal vesicles (ILVs) within MVBs in the endocytic pathway and be released from cells upon the fusion of MVBs with the plasma membrane.24,54,55,56 First, plasma membrane- and cytosol-associated molecules such as nucleic acids, lipids, and proteins are endocytosed and transferred into early endosomes. During the endosome maturation process, early endosomes differentiate into late endosomes/MVBs. Then, late endosomes fuse with lysosomes, leading to their degradation, or fuse with the plasma membrane, releasing the vesicles into the extracellular space as exosomes57,58,59,60 (Fig. 1). The Rab family of small GTPases controls different steps of vesicular trafficking. Rab27a and Rab27b were found to function in MVE docking at the plasma membrane.61 Rab27a has a key role in the size determination of MVEs, whereas Rab27b mediates the transfer of MVEs from microtubules to the actin-rich cortex and their retention at the cell periphery.62 Ultimately, exosomes interact with recipient cells by direct signaling through ligand/receptor molecules on their respective surfaces or are taken up by recipient cells in unique fashion, such as direct membrane fusion, endocytosis, macropinocytosis, or even phagocytosis.8,63,64,65,66,67,68,69
Exosome biogenesis and composition. Exosomes originate not only from ILVs in MVBs but also from plasma membrane budding. Early endosomes maturate into late endosomes/MVBs, which follow either the secretory or the degradative pathway. Microvesicles are generated by budding from the cytomembrane. Apoptotic bodies are generated during programmed cell death (left). Exosomes have spherical structures consisting of a lipid bilayer and contain complex contents, including proteins, mRNA, miRNA, ncRNA, and DNA (right)
The exosome generation pathway can be regulated by either the endosomal sorting complex required for transport (ESCRT)-dependent pathway or an ESCRT-independent pathway.70 In the typical case, ESCRT machinery is required for MVB formation because it sorts ubiquitinated intracellular cargos that are destined for lysosomal degradation into MVBs.71 The ESCRT machinery consists of four ESCRT proteins (ESRT-0, ESRT-I, ESRT-II, and ESRT-III) and accessory proteins (VPS4, VTA1, and the ALG2-interacting protein X (ALIX) complex).72 Whereas the ESCRT-0 subunit, hepatocyte growth factor-regulated tyrosine kinase substrate (HRS), binds ubiquitylated proteins, ESCRT-I (comprising tumor susceptibility gene 101 protein (TSG101) and Vps28) is recruited to the endosomal membrane and incorporates ESCRT-II (Vps22).73,74,75 ESCRT-I and ESCRT-II then facilitate the formation of reverse budding in MVB membranes and uptake of cytosolic cargo.76 Next, ESCRT-II recruits ESCRT-III to catalyze vesicle cleavage inside the neck of nascent ILVs.75,76,77 Although these ESCRT subunits are released into the cytosol for recycling, some ESCRT components and accessory proteins, such as TSG101, HRS, and ALIX, remain in exosomes. In general, the ESCRT machinery is predominantly involved in the sorting of ubiquitinylated proteins into ILVs.70,78 However, not all proteins require ubiquitylation to be sorted into exosomes. In addition to the ESCRT-dependent formation of MVBs and exosomes, ESCRT-independent mechanisms of exosome formation and release were found to depend on neutral sphingomyelinase (nSMase)-dependent ceramide formation.79 Thus, using the inhibitor GW4869, which blocks the generation of ceramide, has been shown to reduce the release of exosomes.79 In addition, another mechanism of ESCRT-independent endosomal sorting involves tetraspanins (such as CD9, CD63, CD81, and CD82), which mediate the organization of particular proteins.80 Presumably, these two alternative mechanisms of MVB and exosome biogenesis do not operate independently and may coexist within a cell or subset population of MVBs.
Exosome composition
Exosomes are heterogeneous and carry plasma membrane- and cytosol-associated molecules such as nucleic acids, lipids, and proteins both inside and outside the vesicles (Fig. 1). As mentioned above, exosomes are highly enriched in ESCRT machinery-associated proteins (such as TSG101, HRS, and ALIX), which are involved in MVB synthesis, and tetraspanins (such as CD9, CD63, and CD81), which are frequently recognized as exosome markers.9,81 In addition, various metabolic enzymes, such as ATPase, glyceraldehyde-phosphate dehydrogenase (GAPDH), enolase 1, pyruvate kinase type M2 (PKM2) and phosphoglycerate kinase 1 (PGK1), have been detected in exosomes by proteomic analyses.82,83 Exosomes also include molecules that are involved in signal transduction, such as 14-3-3 and G proteins. Exosomal 14-3-3 can activate the oncogenic Wnt pathway in target colorectal cancer (CRC) cells in vitro.84 Hepatocellular carcinoma-derived exosomal 14-3-3 impairs the antitumor function of tumor-infiltrating T lymphocytes.85 Furthermore, heat shock proteins (such as HSP70 and HSP90) and major histocompatibility complex (MHC) molecules are also found in exosomes derived from most cell types and are involved in antigen presentation.86,87,88 Interestingly, compared with exosomes, exomeres have unique proteins and are highly enriched with metabolic enzymes and signature proteins involved in glycolysis and mTORC1 signaling.7,89 In addition to the abovementioned evolutionarily conserved and commonly found proteins, exosomes also contain proteins that are involved in specific functions. For instance, tumor cell exosomes usually contain both tumor antigens and immunosuppressive proteins. Programmed death-ligand 1 (PD-L1), a membrane-bound ligand on many cancer cells, was found to be specifically enriched on exosomes from plasma samples of patients with a variety of cancers, suggesting circulating exosomal PD-L1 as a biomarker for the clinical outcomes of anti-PD-1 therapy.90,91 In addition to proteins, the exosome membrane contains a total of 1116 lipids, according to the exosome database (www.exocarta.org), such as phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidyl inositols (PIs), phosphatidic acid (PA), cholesterol, ceramides, sphingomyelin, glycosphingolipids, and a number of others at lower abundance.11,12,92 Importantly, lipidomic analyses revealed cell type-dependent differences in the total lipid level and composition among different subpopulations of EVs.7 Future exploration of this issue will undoubtedly contribute to the understanding of exosome formation and secretion and greatly advance the understanding of the tissue/organ specificity of exosome function. Exosomes also contain mRNAs and noncoding RNAs (ncRNAs), including microRNAs (miRNAs), small nuclear RNAs (snRNAs), transfer RNAs (tRNAs) and Y RNAs.93,94 Exosomal RNA transmission can alter the epigenetic characteristics of target cells, largely through gene regulation. An increasing number of studies have indicated that tumor-derived exosomes (TEXs) contain high levels of miRNAs, which are thought to be potential circulating diagnostic biomarkers in many types of cancer, such as glioblastoma, ovarian cancer and prostate cancer.41,95,96 Although several studies have reported the presence of DNA in exosomes, including single-stranded DNA, double-stranded DNA (dsDNA), genomic DNA and even mitochondrial DNA,13,14,15,97 there is an opposite view that suggests that dsDNA is not present in exosomes or any other type of small extracellular vesicle.98 The debate on this issue is likely based on early studies that often did not discriminate between MVs and exosomes. The International Society for Extracellular Vesicles (ISEV) proposed Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines in 2014 and updated it in 2018 to guide and improve the EV field.99,100 In future studies, there is an urgent need to improve the methodology for exosome isolation, which is expected to enable more precise determination of the molecular composition of classical exosomes.
Functions of exosomes in innate immune signaling and regulation
Innate immune responses
In response to infection, host cells sense invading viruses and initiate a series of signaling pathways that lead to the production of type I interferons and the expression of an array of interferon-stimulated genes (ISGs).101,102 The recognition of foreign nucleic acids is a critical strategy by which the innate immune system recognizes many pathogens.103 Several nucleic acid sensors have been identified, including cytosolic RNA sensors, such as TLR3, TLR7, TLR8, RIG-I and MDA5, and DNA sensors, such as TLR9, AIM2, and cGAS.43,48,49,104,105,106,107 Following the recognition of viral nucleic acids, PRRs recruit downstream adaptors, including TRIF, MAVS, and STING, which subsequently activate downstream kinases such as inhibitor IκB kinase (IKK) complexes composed of either IKKα, IKKβ, and IKKγ or kinases TBK1 and IKKε.108,109,110 The IKK complex and TBK1-IKKε activate transcription factors NF-κB and IRF3, which then are translocated to the nucleus, where they are involved in the production of pro-inflammatory cytokines regulated by NF-κB signaling and type I interferons (IFN-α and IFN-β) as mediated by IRF signaling.50,51,52,53 IFN-β and IFN-α subsequently activate downstream signaling pathways that induce a diverse set of interferon-stimulated genes and protect host cells against the invading virus. We summarize the proposed roles of EVs and exosomes containing cargo, including dsDNA, virus RNA or a specific protein, in modulating innate immune responses (Fig. 2).
Functions of exosomes in innate immunity. a Activated T cell-derived exosomes containing DNA are transferred to DCs, inducing an antiviral IFN response; RNA-bearing exosomes secreted by virus-infected cells activate the innate immune system of DCs; and irradiated cancer cells deliver tumor dsDNA to DCs via exosomes, leading to DC activation and IFN I release. b Tumor cells secrete and transfer EGFR+ exosomes to macrophages, which interfere with innate antiviral immunity via MEKK2-mediated deregulation of IRF3, and tumor-derived microvesicles/exosomes containing a ligand for NKG2D downregulate NKG2D expression on DCs and inhibit the cytotoxic activity of DCs. c ODN-loaded extracellular vesicles derived from TLR9-activated macrophages are transported to naïve macrophages and induce the release of chemokine TNF-α; tumor-secreted miR-21 and miR-29a bind with TLR7 and TLR8 in macrophages, triggering a prometastatic inflammatory response; and TEXs containing HSP70/HSP72 activate NF-κB signaling through TLR2 on MDSCs or MSCs
EV-mediated innate immune response activation
Consistent with previous reports, dsDNA or virus RNA-containing EVs or exosomes can trigger immune responses.111,112,113,114 Upon activation by interactions with antigen-bearing dendritic cells, T cells transmit EVs that contain genomic and mitochondrial DNA back to the presenting DCs, further enhancing antiviral responses via the cGAS/STING cytosolic DNA-sensing pathway and the subsequent induction of IRF3-dependent interferon-regulated genes in vitro111 (Fig. 2a). These results suggest a feedback mechanism by which T cells enhance the activity of an antigen-presenting cell (APC), priming it to respond more efficiently to subsequent infections by the same pathogen or a similar pathogen. As the changes in DCs induced by T cell-derived EVs occur in a specific antigen-dependent manner, the enhanced innate antiviral immunity triggered by DCs responds only to specific stimuli. Interestingly, oxidized mtDNA and genomic DNA, together with mtDNA-binding proteins, are present in T cell exosomes, suggesting that these EVs may maintain cellular homeostasis by releasing harmful or damaged components.111,115 However, the evidence that signaling mediates the loading of DNA into late endosomes/MVBs is lacking, and thus further investigation is warranted. In addition to immune cells, cancer cells also release DNA-loaded exosomes/EVs under certain conditions, such as during radiation treatment or chemotherapy. For example, a recent study showed that treatment of breast cancer cells with the topoisomerase I inhibitor topotecan, an antitumor chemotherapy, significantly increased exosomal DNA production, which led to the activation of dendritic cells through cGAS-STING signaling in vitro and in vivo.116 This finding suggests that exosomal DNA can also activate innate antiviral immune cell responses.116,117 Interestingly, it was recently noticed that combinations of radiotherapy and immunotherapy can lead to more effective antitumor responses and the reasons for this synergy in cancer treatment has attracted attention.118,119 Radiotherapy destroys cancer cells in the area where it is applied. Although normal cells in the area can also be damaged by radiotherapy, they are usually able to repair themselves, but cancer cells cannot undergo self-repair. Radiotherapy induces immunogenic cell death and the release of new antigens into the immune system, thereby affecting the immune response and improving the initiation and activation of effector T cells. At immunogenic doses, radiotherapy causes the accumulation of cytosolic dsDNA in cancer cells, which is sensed by cyclic GMP-AMP synthase (cGAS) in DCs, resulting in the production of IFN-β and the induction of several interferon-stimulated genes (ISGs) in vitro and in vivo112 (Fig. 2a). EVs from cancer cell lines and patients contain DNA that reflects the mutational status of the parental tumor cells.13,15 Irradiated cancer cells thus also release exosomes that carry the tumor dsDNA to the cytosol of DCs, leading to DC activation and antitumor T cell priming.112 However, the following hypothesis needs further investigation: circulating TEXs in peripheral blood may be biomarkers that indicate RT-induced immunogenic changes in tumors. In fact, these findings provide a theoretical basis for suggesting a new vaccination strategy.
It is well known that viral nucleic acids often trigger an innate immune response in infected cells. In addition to DNA, various types of RNA have been identified in exosomes.114 For example, latent Epstein–Barr virus (EBV)-infected cells can trigger antiviral immunity through dendritic cells (DCs) by the exosomal transfer of 5’ppp-RNA in vitro.114 This finding suggests that 5’ppp-recognizing sensors such as RIG-I are more likely to have a role in the recognition of exosomal small RNA. Consistent with this report, exosomes derived from breast cancer stromal fibroblasts carry 5’ppp-RNA RN7SL1, which activates RIG-I in tumor cells and results in STAT1 activation and ISG induction in vivo and in vitro113 (Fig. 2a). Therefore, a conserved intercellular pathway transmits signals between cells in the form of small RNAs via exosomes. Upon recognizing invading viruses, host cells trigger signaling events that ultimately lead to type I interferon secretion. Despite the mechanism by which many viruses evade the pathogen-sensing pathway, there are alternative pathogen-sensing strategies that are not challenged by viral evasion mechanisms. For example, hepatitis C virus (HCV)-permissive cells can selectively package immunostimulatory viral RNA into exosomes and deliver it to neighboring plasmacytoid DCs (pDCs), which triggers an antiviral IFN response in vitro.120 This finding describes a mechanism that was previously undiscovered in innate host responses, whereby infected cells with a pathogen-sensing mechanism that is inhibited by viral proteins can release viral RNA-containing exosomes to trigger an alternative host defense strategy.
EV-induced innate immunosuppression
Emerging evidence has shown that tumors can interfere with host immunity by secreting EVs or exosomes. By transducing different signals, TEXs can affect the proliferation, apoptosis, cytokine production and reprogramming of both innate and adaptive immune cells.121,122,123 For example, TEXs enriched with miRNAs, such as miR-21-3p, miR-125b-5p, miR-181d-5p and miR-1246, potently reprogram neighboring macrophages into tumor-supportive agents.124,125 Previous studies suggested that tumor-derived microvesicles/exosomes expressing TGF-β1 and a ligand for NKG2D can downregulate NKG2D expression and reduce the cytotoxicity induced by natural killer cells126,127,128 (Fig. 2b). In addition, tumor-derived microvesicles/exosomes can also promote the differentiation of monocytes into myeloid-derived suppressor cells (MDSCs), which inhibit DC maturation.129,130,131
Epidermal growth factor receptor (EGFR), located on the cell membrane, belongs to the ErbB family of receptor tyrosine kinases (RTKs).132,133,134 Highly or abnormally expressed EGFR is associated with the occurrence of various kinds of tumors.135 In the tumor microenvironment, TEX-mediated delivery of EGFR and human epidermal growth factor receptor 2 (HER-2) to monocytes promotes tumor-derived monocyte survival prior to the formation of numerous tumor-associated macrophages (TAMs).136 In line with this report, it was suggested that ALIX, a critical mediator of exosome biogenesis, modulates immunosuppression through the regulation of PD-L1 and EGFR in breast cancer cells.137,138 ALIX depletion results in enhanced EGFR activity as well as reduced exosomal PD-L1 secretion and increased surface PD-L1 expression.137 However, a recent study showed that exosomal PD-L1 contributes to immunosuppression in patients with metastatic melanoma.90 Chronic lymphocytic leukemia (CCL)-derived exosomes and the Y RNA they contain can also induce PD-L1 expression and cytokine release in monocytes and thus contribute to a tumor-supportive microenvironment.139 Similarly, EGFR is in the exosomes secreted by gastric cancer cells and is highly expressed in the exosomes of cancer patients; by stimulating paracrine HGF in liver stromal cells, EGFR-containing exosomes derived from gastric cancer cells may favor the development of a liver-like microenvironment promoting liver-specific metastasis in vitro and in vivo.140 Recently, our group discovered previously unknown interplay between lung tumor cells and innate antiviral immunity in virus-infected mice by elucidating a TEX-mediated control mechanism of IRF3 signaling in recipient macrophages in vitro and in vivo.141 By secreting and transferring EGFR+ exosomes to the host macrophages, tumor-derived EGFR stimulated host MEKK2, which unexpectedly phosphorylated IRF3 at Ser173; this modification led to the repression of IRF3 and type I interferon activation, weakening the host’s pathogen-defense ability (Fig. 2b). The results from this study explained a process by which malignant tumors can interfere with the innate antiviral system via exosomes and identified a mechanism by which cancer cells can dampen host innate immunity. In addition, these mechanistic studies helped to explain the diminished innate antiviral immunity frequently found in patients with cancer.142
Indeed, EV-innate immune system cross talk may evolve and thus may differ during tumor development. In our opinion, the functions of exosomes in cancer are determined by their specific cargos. Under certain physiological conditions or in a primary tumor, premetastatic niche and metastatic tumor sites, different TEXs containing specific contents may mediate either immunostimulatory or immunoinhibitory activity. As explained above, TEXs carrying tumor antigens can be transferred to DCs and elicit tumor-specific immune responses.143 However, in most cases, TEXs containing immunosuppressive proteins such as PD-L1 and EGFR have been shown to cause innate immunosuppression and protumorigenic effects.90,91,141,144
EV-mediated regulation of TLR/NF-κB signaling
TLRs, a class of proteins that play key roles in the innate immune system, were the earliest discovered and most well-studied PRRs.145 There are 11 TLRs (TLR1-11) that have been identified in humans, and these different TLRs specifically recognize distinct PAMPs and DAMPs.146,147,148,149 TLR3, TLR7, TLR8 and TLR9 sense viral RNA and DNA in the endosome.47,101,150 Among these TLRs, TLR9 recognizes CpG-containing oligodeoxynucleotides (CpG-ODNs), and the TLR9 agonist synthetic CpG-ODN is being investigated as a cancer vaccine adjuvant in clinical trials.151,152 TLR3 in lung epithelial cells can be activated by exosomal small nuclear RNA secreted by primary tumors, resulting in the production of chemokines and the recruitment of neutrophils.153 Once recruited in the premetastatic niche, neutrophils were shown to elicit a prometastatic inflammatory microenvironment by suppressing both innate and adaptive antitumor immunity.154,155,156,157 Gastric cancer cell-derived exosomes containing high mobility group box-1 (HMGB1) also induce the tumor-promoting activation of neutrophils, via TLR4/NF-κB signaling.158
Recent studies demonstrated that EVs secreted by immune cells can also play immune regulatory roles.159,160,161 For example, exosomes derived from mycobacteria-infected macrophages contain TLR ligands and thus can promote both innate and acquired immune responses.159 Microvesicles released from infected red blood cells activate host monocytes and neutrophils.161 In line with this finding, TLR9-activated macrophages transport ODN to naïve macrophages via EVs, thus inducing the release of chemokine TNF-α, resulting in a synergetic effect in the propagation of the intracellular immune response in vitro162 (Fig. 2c). This study also elucidated the role of EVs in the internalization of different PAMPs and the subsequent activation of intracellular innate immune signaling. In addition to ODN, Cdc42 is transferred from EVs into naïve macrophages and further activated by TNF-α, leading to the enhancement of EV uptake.162 Similar to TLR9, TLR7 and TLR8 localize to intracellular compartments such as endosomes, lysosomes and the ER.150,152,163 Exosomal miR-21 and miR-29a secreted by lung cancer cells in BALB/c mice were transferred to macrophages where they could bind to TLR7/8, leading to TLR-mediated NF-κB activation and secretion of the prometastatic inflammatory cytokine TNF-α in vitro and in vivo164 (Fig. 2c). These results suggest that the transfer of EVs with nucleic acids can activate TLR molecules to initiate innate immune responses. In addition to nucleic acids, proteins such as HSP72, which has been found on the surface of TEXs, can also activate NF-κB signaling and induce the production of interleukin-6 and TNF-α in myeloid-derived suppressor cells (MDSCs) in a TLR2/MyD88-dependent manner165,166 (Fig. 2c). Similar results showed that TEXs that contained HSP70 activated NF-κB signaling through TLR2 on mesenchymal stem cells (MSCs) in vitro and in vivo in nude mice.167 During cancer progression, exosomes derived from breast cancer cells were reported to induce the secretion of TLR2- and TLR4-dependent pro-inflammatory factors by distant macrophages168,169 (Fig. 2c). In summary, the latest evidence has revealed that TLR signaling can be the target of EVs and is required for EV-induced NF-κB activation, as well as the production of pro-inflammatory cytokines. However, we must recognize that the study of the EV-mediated regulation of TLR/NF-κB is still in its infancy; how this process is tightly controlled remains unknown. Future studies with more detailed elucidation of EV composition will help to reveal the intricate mechanisms of EV-induced immunomodulation.
Exosomes in cancer diagnostic and therapeutic applications
Liquid biopsy based on exosomes
Currently, liquid biopsy has emerged as a noninvasive and convenient approach for cancer diagnosis and prognostic monitoring. In contrast to surgical biopsy and puncture biopsy, liquid biopsy can be used to directly detect circulating tumor cells (CTCs), circulating tumor DNA (ctDNA) or cell-free tumor RNA from blood, saliva and other body fluids.
More recently, exosomes have become particularly valued for use in liquid biopsy because of their natural advantages over other samples.170 For example, exosomes can protect nucleic acids from rapid degradation; the formation of exosomes is closely related to the state of parental cells; the detection of exosomes is more specific than that of traditional tumor markers; and exosomes are widely found in various body fluid samples. Compared with CTCs, exosomes circulate at higher concentrations in blood (e.g., >109 vesicles per mL of blood); therefore, only a small volume of blood is necessary for analysis.170 Moreover, exosomes are highly stable in blood plasma, whereas ctDNA and cell-free tumor RNA are rapidly degraded. The standardization of sample collection, isolation and analysis methods for exosome isolation from small amounts of biofluids, such as blood plasma, has been reported in several previous ISEV position papers.99,171,172 Ultracentrifugation is the most traditional and widely accepted technology used for exosome purification from blood plasma or cell culture supernatants.6,173,174 Exosome-based diagnosis can also be used to monitor changes in molecular markers over time during the development of the disease. Owing to the ease and noninvasive nature of sample collection, exosome-based liquid biopsy provides clinical information, including that for diagnosis and prognosis, which contributes to clinical decisions (e.g., precision or personalized therapy, disease monitoring)175 (Fig. 3a).
Exosomes in cancer diagnostic and therapeutic applications. a Exosomes bearing certain proteins, miRNAs or DNA may be valuable as cancer biomarkers in liquid biopsy samples. The test results may provide meaningful guidance for disease screening, prognosis, diagnosis, risk assessment, clinical decisions, and personalized treatment. b Exosomes derived from M1-polarized macrophages or NK cells may be used as vaccine adjuvants, and radiation-induced tumor DNA-loaded exosomes or antigen-loaded DC exosomes are potential vaccines for cancer treatment
Exosomal nucleic acids, including microRNAs (miRNAs), mRNA, lncRNA, and DNA, are involved in cancer angiogenesis and metastasis and may be promising biomarkers for cancer diagnosis13,15,176 (Fig. 3a). Over the past decades, various miRNAs in exosomes have been shown to be potential biomarkers for various types of cancer, including lung cancer, liver cancer, gastrointestinal cancer, pancreatic cancer, melanoma, breast cancer, ovarian cancer, and prostate cancer. For example, oncogenic mutated KRAS (KRAS G12D and KRAS G12V) mRNAs have been detected in serum exosomes of patients with pancreatic cancer. Interestingly, circular RNAs (circRNAs) were also abundant in exosomes derived from cancer cells and patient serum, and they may serve as a new class of exosome-based cancer biomarkers.177,178 In addition to nucleic acids, exosomes also contain a variety of protein molecules that reflect the characteristics of their parental cells and thus can be used as molecular markers for tumor diagnosis. Although a large number of serum samples from pancreatic cancer patients showed that the proportion of glypican-1 (GPC1)-positive exosomes in the serum of pancreatic cancer patients was significantly higher than that in healthy patients,179 the clinical application of GPC1-positive exosomes is still controversial. Some have voiced concern about diagnoses proclaimed to have 100% sensitivity, specificity, positive and negative predictive value.180,181 Thus, it remains to be seen whether GPC1-carrying exosomes will be subsequently validated by other research groups. Recently, high GPC1 crExos were used to determine PDAC tumor size and disease burden, but they could not distinguish PDAC from benign pancreatic disease at the GPC1 levels carried by the crExos182. Furthermore, the combined detection of exosomal GPC1, exosomal CD82, and serum CA19-9 shows great promise as a standard method for PC detection.183 Another study has shown that the determination of S100B and MIA in exosomes may be an alternative to their analysis in serum for the diagnosis and prognosis of melanoma patients.184 In addition, exosomal cytoskeleton-associated protein 4 (CKAP4), a novel DKK1 receptor, may represent a biomarker for pancreatic cancer.185 Therefore, these methods are claimed to accurately diagnose and distinguish early cancer, which may substantially change the fate of patients with cancer. However, little data concerning the expression of specific cancer-associated biomarkers for monitoring the outcome of their use in cancer patients are available, and data on prostate cancer and melanoma have been published without further clinical application.184 The application of exosome-based cancer therapies is urgently needed.
Application of exosomes in cancer vaccines
Due to their high stability in circulation and low toxicity and immunogenicity, exosomes for use as vehicles in clinical practice are promising, and the idea of using them is inspiring.186,187 Modified exosomes as drug delivery carriers loaded with tumor drugs or tumor-targeting RNAi have been designed for clinical applications.188,189,190 In addition, as a typical immunotherapy for tumors, cancer vaccines are research hotspots that have increasingly attracted attention in recent years, and their clinical application has recently been greatly improved.191,192,193,194 Cancer vaccines are most suitable for cancer patients receiving early or postradical treatment rather than patients with advanced disease. As mentioned above, exosomes derived from virus-infected cells can trigger an antiviral IFN response, which makes them attractive candidates for cancer vaccine development. NK cells are innate lymphoid cells that play a central role in the immune response against cancer.195 Results from a recent study indicated that NK cell-derived exosomes mediate antitumor effects against aggressive melanoma in vitro and in vivo.196 In addition, exosomes derived from M1-polarized macrophages may be used as a vaccine adjuvant.197 Recently, engineered exosomes have emerged as novel approaches for cancer vaccine development in immunotherapy and have been applied in phase I clinical trials.198 Large-scale production and purification of clinical grade exosomes from dendritic cells using good manufacturing practice have been reported, and these exosomes were found to enhance NK cell function in patients with non-small cell lung carcinoma or melanoma.191,194 Dendritic cell-derived exosomes (DEXs) loaded with synthetic CTL-defined epitopes, such as melanoma antigen recognized by T cells 1 (MART1)26–35 peptides, by nanotechnology were shown to elicit stronger immune responses toward cancer cells.198 A phase II clinical trial tested the clinical benefit of IFN-γ-DEX loaded with MHC I/II-restricted cancer antigens as maintenance immunotherapy after induction chemotherapy.199 However, a T cell response was not found in patients bearing inoperable non-small cell lung cancer (NSCLC) without tumor progression.199 One reason for the limited efficacy for DEX immunotherapy is that the INF-γ used in the process of DEX production may upregulate PD-L1, an inhibitor of T-cell activation, in DCs and DEXs.200,201,202 Previous studies have shown that PD-L1 is a marker of the immunosuppressive DC subset that accumulates as tumors progress.203,204,205,206 Recent findings indicated that exosomal PD-L1 derived from cancer cells contributes to immunosuppression and mediates immune evasion.90,91 It remains to be determined whether DEXs express functional PD-L1 or PD-L2 molecules that may restrict T cell responses. Therefore, using DCs with low PD-L1 expression to generate DC-based vaccines may be a strategy against cancer. Indeed, in line with this possibility, combining DC-based vaccines with the suppression of inhibitory signals, as with a PD-1/PD-L1 blockade or anti-CTLA4 therapy, has great potential for eliciting a better immune response against cancer.207,208 Another study showed that targeting ovalbumin (OVA) and the G protein of vesicular stomatitis virus (VSV-G) to the same exosome-like vesicles improved the immunogenicity of exosomal vaccines in vivo.209 Thus, incorporation of a viral fusion protein and targeting of antigens to DEXs are attractive strategies to enhance the immunogenicity of exosomal vaccines.209 Therefore, as a new vaccine strategy for cancer immunotherapy, DEX remains promising with potential for improvement. It is important to combine DC-based vaccines with new approaches that overcome the immunosuppressive mechanisms in the tumor microenvironment and promote the activation of the immune system (Fig. 3b).
Discussion
Over the past decade, the field of EVs, including that studying exosomes, has emerged as an exciting area of research. The evidence gathered from various sources has revealed that exosomes play unexpected functions in broad biological processes, including those of human diseases, such as antigen presentation, immune response, cancer metastasis, inflammation and drug resistance, through intercellular communication.28,123,210,211,212,213,214,215 Although some breakthroughs have been made, many more unknown areas have emerged, and an increasing number of technical problems need to be resolved. Although the biogenesis of EVs or exosomes is relatively clear, the sorting and screening of exosome contents are usually linked to the ESCRT complex, and the delivery process is less clear. Questions abound on how specific proteins, RNAs and even DNA are selectively packaged into exosomes, and answers are needed for this apparent, urgent problem in the basic research field of EVs. Another inspiring question remains elusive: why do exosomes secreted by different cell types always play roles, albeit diverse, in mediating the innate immunity of recipient cells? EV populations are heterogeneous;7,98,216,217,218,219 without doubt, the functions of exosomes must depend on the specific cargos loaded. It is conceivable that even exosome with identical content could be delivered to different recipient cells to produce different biochemical reactions and effects. In fact, it is an urgent task to comprehensively map the composition of exosomes from different origins, and great effort is required to achieve a consensus about the biochemical definition and classification of EV subpopulations and to determine the cellular signals or events that determine their size and cargo composition.
Currently, research in the field of EVs or exosomes is mainly focused on RNA. However, the mechanisms of loading and transduction, the fate after uptake and the working mode of exosomal RNA are still unclear. Considering that extracellular vesicles carry not only RNA but also a variety of proteins, lipids, and cell metabolites, most scholars believe that EV-associated components have corresponding functions as a whole structure, which definitely warrants further investigation. Interestingly, in a very recent study, adipocytes were found to release intact triglycerides packaged into small particles, called adipocyte exosomes (AdExos).220 The uptake of these AdExos by macrophages in adipose tissue enabled the direct transfer of lipids, revealing a manner of EV-mediated exchange of signals and nutrients between adipocytes, immune cells, and metabolic organs.
By studying EVs in different disease states, we may find how their contents can modulate immune cell function to influence cancer progression. It would be very helpful to summarize the models and technologies currently used for EV-immune study and discuss their limitations. To date, most exosome studies in vitro have been established using a cell coculturing system to investigate the mechanism of EV delivery, uptake, transfer and regulation in immunity. The research of EV-immune system cross talk in vivo is mainly based on well-established transgenic or orthotopic mouse models under certain physiologically relevant experimental conditions. The use of other animal experiments, such as Drosophila or zebrafish models, to study the biogenesis, trafficking and cellular entry of EVs in vivo is currently being considered.221 Another limitation of the current methods used to study EV-immune mechanisms likely involves the collection of pathological samples, which cannot reflect the dynamic changes in EVs. For example, the purification of EVs using ultracentrifugation methods takes a long time in the clinical setting. Therefore, novel models and analytical methods are being developed to improve the ability to characterize the behavioral dynamics and importance of these vesicles in different biological contexts. In addition, it is important to record the information obtained in samples from biofluids because donor age, diet, body mass index, medications, and other factors may affect the contexts of the EV samples. Because of the complexity of the composition and functional heterogeneity of EVs, single-vesicle identification, isolation and analyses are recommended, which will substantially accelerate our understanding of EV biology, EV-based therapies, and diagnostics. The application of exosomes as immunotherapeutics for cancer is promising, especially the use of exosomes derived from immune cells. Although studies have suggested that EVs are the main media of intercellular communication in the immune system, clinical trials have shown that using immune cell-derived EVs alone is often insufficient to induce an effective immune response in vivo.222 To boost their immunogenicity, engineered exosomes with tumor antigens are being generated to make them more recognizable by the immune system.223 Thus, the targeting specificity of EVs needs to be understood, and an effective strategy for loading nucleic acids, proteins and/or lipids into EVs needs to be developed. Although more work is definitely required to understand the complex functions of exosomes in innate immune regulation, their mysteries will eventually be unveiled.
References
Thery, C., Ostrowski, M. & Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
Simons, M. & Raposo, G. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581 (2009).
Pegtel, D. M. & Gould, S. J. Exosomes. Annu Rev. Biochem 88, 487–514 (2019).
Daaboul, G. G. et al. Digital detection of exosomes by interferometric imaging. Sci. Rep. 6, 37246 (2016).
Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002).
Xu, R. et al. Extracellular vesicle isolation and characterization: toward clinical application. J. Clin. Invest 126, 1152–1162 (2016).
Zhang, H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332–343 (2018).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Hegmans, J. P. J. J. et al. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am. J. Pathol. 164, 1807–1815 (2004).
Bard, M. P. et al. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am. J. Respir. Cell Mol. Biol. 31, 114–121 (2004).
Skotland, T., Sandvig, K. & Llorente, A. Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res 66, 30–41 (2017).
Llorente, A. et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys. Acta 1831, 1302–1309 (2013).
Thakur, B. K. et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24, 766–769 (2014).
Balaj, L. et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180 (2011).
Kahlert, C. et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 289, 3869–3875 (2014).
Pisitkun, T., Shen, R. F. & Knepper, M. A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl Acad. Sci. USA 101, 13368–13373 (2004).
Admyre, C. et al. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179, 1969–1978 (2007).
Michael, A. et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral. Dis. 16, 34–38 (2010).
Hiemstra, T. F. et al. Human urinary exosomes as innate immune effectors. J. Am. Soc. Nephrol. 25, 2017–2027 (2014).
Zijlstra, C. & Stoorvogel, W. Prostasomes as a source of diagnostic biomarkers for prostate cancer. J. Clin. Invest 126, 1144–1151 (2016).
Trams, E. G. et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys. Acta 645, 63–70 (1981).
Fang, Y. et al. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 5, e158 (2007).
Booth, A. M. et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J. Cell Biol. 172, 923–35. (2006).
Denzer, K. et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113(Pt 19), 3365–3374 (2000).
Keller, S. et al. Exosomes: from biogenesis and secretion to biological function. Immunol. Lett. 107, 102–108 (2006).
Deneka, M. et al. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J. Cell Biol. 177, 329–341 (2007).
Casado, S., Lobo, M. & Paino, C. L. Dynamics of plasma membrane surface related to the release of extracellular vesicles by mesenchymal stem cells in culture. Sci. Rep. 7, 6767 (2017).
Becker, A. et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848 (2016).
Robbins, P. D. & Morelli, A. E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14, 195–208 (2014).
Maus, R. L. G. et al. Human melanoma-derived extracellular vesicles regulate dendritic cell maturation. Front Immunol. 8, 358 (2017).
Capello, M. et al. Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat. Commun. 10, 254 (2019).
Minciacchi, V. R., Freeman, M. R. & Di Vizio, D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev. Biol. 40, 41–51 (2015).
Di Vizio, D. et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res 69, 5601–5609 (2009).
Minciacchi, V. R. et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 6, 11327–11341 (2015).
Minciacchi, V. R. et al. MYC mediates large oncosome-induced fibroblast reprogramming in prostate cancer. Cancer Res 77, 2306–2317 (2017).
Morello, M. et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 12, 3526–3536 (2013).
Di Vizio, D. et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 181, 1573–1584 (2012).
Dell’angelica, E. C. et al. Lysosome-related organelles. FASEB J. 14, 1265–1278 (2000).
Marks, M. S. & Seabra, M. C. The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2, 738–748 (2001).
Rivoltini, L. et al. Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol. Rev. 188, 97–113 (2002).
Taylor, D. D. & Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13–21 (2008).
Shaw, A. C. et al. Aging of the innate immune system. Curr. Opin. Immunol. 22, 507–513 (2010).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Alexopoulou, L. et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Barrat, F. J., Elkon, K. B. & Fitzgerald, K. A. Importance of nucleic acid recognition in inflammation and autoimmunity. Annu Rev. Med 67, 323–336 (2016).
Hornung, V. et al. OAS proteins and cGAS: unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 14, 521–528 (2014).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Paludan, S. R. et al. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 11, 143–154 (2011).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Takeuchi, O. & Akira, S. Innate immunity to virus infection. Immunol. Rev. 227, 75–86 (2009).
Mathieu, M. et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev. Cell Dev. Biol. 30, 255–289 (2014).
Kowal, J., Tkach, M. & Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 29, 116–125 (2014).
Stoorvogel, W. et al. The biogenesis and functions of exosomes. Traffic 3, 321–330 (2002).
Baietti, M. F. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685 (2012).
Buschow, S. I. et al. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 88, 851–856 (2010).
Bowers, K. & Stevens, T. H. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim. Biophys Acta. 1744, 438–454 (2005).
Zerial, M. & Mcbride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117 (2001).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30 (2010). sup pp 1-13.
Nanbo, A. et al. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J. Virol. 87, 10334–10347 (2013).
Svensson, K. J. et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 288, 17713–17724 (2013).
Escrevente, C. et al. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 11, 108 (2011).
Feng, D. et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic 11, 675–687 (2010).
Morelli, A. E. et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104, 3257–3266 (2004).
Fitzner, D. et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458 (2011).
Tkach, M. & Thery, C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 (2016).
Shields, S. B. et al. ESCRT ubiquitin-binding domains function cooperatively during MVB cargo sorting. J. Cell Biol. 185, 213–224 (2009).
Colombo, M. et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 126(Pt 24), 5553–5565 (2013).
Gill, D. J. et al. Structural insight into the ESCRT-I/-II link and its role in MVB trafficking. EMBO J. 26, 600–612 (2007).
Tamai, K. et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys. Res Commun. 399, 384–390 (2010).
Flores-Rodriguez, N. et al. ESCRT-0 marks an APPL1-independent transit route for EGFR between the cell surface and the EEA1-positive early endosome. J. Cell Sci. 128, 755–767 (2015).
Babst, M. et al. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3, 271–282 (2002).
Babst, M. et al. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3, 283–289 (2002).
Buchkovich, N. J. et al. Essential N-terminal insertion motif anchors the ESCRT-III filament during MVB vesicle formation. Dev. Cell 27, 201–214 (2013).
Katzmann, D. J., Babst, M. & Emr, S. D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106, 145–155 (2001).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Van Niel, G. et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 21, 708–721 (2011).
Mathivanan, S. et al. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol. Cell Proteom. 9, 197–208 (2010).
Poliakov, A. et al. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate 69, 159–167 (2009).
Conde-Vancells, J. et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J. Proteome Res. 7, 5157–5166 (2008).
Dovrat, S. et al. 14-3-3 and beta-catenin are secreted on extracellular vesicles to activate the oncogenic Wnt pathway. Mol. Oncol. 8, 894–911 (2014).
Wang, X. et al. 14-3-3zeta delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 9, 159 (2018).
Utsugi-Kobukai, S. et al. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol. Lett. 89, 125–131 (2003).
Muntasell, A., Berger, A. C. & Roche, P. A. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 26, 4263–4272 (2007).
Gastpar, R. et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 65, 5238–5247 (2005).
Zhang, Q. et al. Transfer of functional cargo in exomeres. Cell Rep. 27, 940–954 e6 (2019).
Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 (2018).
Poggio, M. et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 177, 414–427 (2019).
Mathivanan, S. et al. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40, D1241–D1244 (2012).
Shurtleff, M. J. et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc. Natl Acad. Sci. USA 114, E8987–E8995 (2017).
Wei, Z. et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 8, 1145 (2017).
Bryant, R. J. et al. Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer 106, 768–774 (2012).
Manterola, L. et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro Oncol. 16, 520–527 (2014).
Sansone, P. et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl Acad. Sci. USA 114, E9066–E9075 (2017).
Jeppesen, D. K. et al. Reassessment of exosome composition. Cell 177, 428–445 (2019). e18.
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Lotvall, J. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 3, 26913 (2014).
Wu, J. & Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev. Immunol. 32, 461–488 (2014).
Kato, H., Takahasi, K. & Fujita, T. RIG-I-like receptors: cytoplasmic sensors for non-self RNA. Immunol. Rev. 243, 91–98 (2011).
Barbalat, R. et al. Nucleic acid recognition by the innate immune system. Annu Rev. Immunol. 29, 185–214 (2011).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Sun, L. et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Lund, J. M. et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl Acad. Sci. USA 101, 5598–5603 (2004).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Seth, R. B. et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, 669–682 (2005).
Sato, S. et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171, 4304–4310 (2003).
Torralba, D. et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 9, 2658 (2018).
Diamond, J. M. et al. Exosomes shuttle TREX1-Sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol. Res. 6, 910–920 (2018).
Nabet, B. Y. et al. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 170, 352–366 (2017). e13.
Baglio, S. R. et al. Sensing of latent EBV infection through exosomal transfer of 5’pppRNA. Proc. Natl Acad. Sci. USA 113, E587–E596 (2016).
Takahashi, A. et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 8, 15287 (2017).
Kitai, Y. et al. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J. Immunol. 198, 1649–1659 (2017).
Kurywchak, P., Tavormina, J. & Kalluri, R. The emerging roles of exosomes in the modulation of immune responses in cancer. Genome Med 10, 23 (2018).
Vacchelli, E. et al. Trial Watch: Immunotherapy plus radiation therapy for oncological indications. Oncoimmunology 5, e1214790 (2016).
Demaria, S., Coleman, C. N. & Formenti, S. C. Radiotherapy: changing the game in immunotherapy. Trends Cancer 2, 286–294 (2016).
Dreux, M. et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 12, 558–570 (2012).
Wieckowski, E. U. et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 183, 3720–3730 (2009).
Whiteside, T. L. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc. Trans. 41, 245–51. (2013).
Whiteside, T. L. Exosomes and tumor-mediated immune suppression. J. Clin. Invest 126, 1216–1223 (2016).
Chen, X. et al. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 435, 80–91 (2018).
Cooks, T. et al. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat. Commun. 9, 771 (2018).
Szczepanski, M. J. et al. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 96, 1302–1309 (2011).
Clayton, A. et al. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 180, 7249–7258 (2008).
Lundholm, M. et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS ONE 9, e108925 (2014).
Valenti, R. et al. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 67, 2912–2915 (2007).
Valenti, R. et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res 66, 9290–9298 (2006).
Yu, S. H. et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J. Immunol. 178, 6867–6875 (2007).
Normanno, N. et al. The role of EGF-related peptides in tumor growth. Front Biosci. 6, D685–D707 (2001).
Yarden, Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 37, S3–S8 (2001).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001).
Pedersen, M. W. et al. The type III epidermal growth factor receptor mutation. Biological significance and potential target for anti-cancer therapy. Ann. Oncol. 12, 745–760 (2001).
Song, X. et al. Cancer cell-derived exosomes induce mitogen-activated protein kinase-dependent monocyte survival by transport of functional receptor tyrosine kinases. J. Biol. Chem. 291, 8453–8464 (2016).
Monypenny, J. et al. ALIX regulates tumor-mediated immunosuppression by controlling EGFR activity and PD-L1 presentation. Cell Rep. 24, 630–641 (2018).
Bissig, C. & Gruenberg, J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 24, 19–25 (2014).
Haderk, F. et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci. Immunol. 2, eaah5509 (2017).
Zhang, H. et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 8, 15016 (2017).
Gao, L. et al. Tumor-derived exosomes antagonize innate antiviral immunity. Nat. Immunol. 19, 233–245 (2018).
Chemaly, R. F. et al. A multicenter study of pandemic influenza A (H1N1) infection in patients with solid tumors in 3 countries: early therapy improves outcomes. Cancer 118, 4627–4633 (2012).
Wolfers, J. et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med 7, 297–303 (2001).
Daassi, D., Mahoney, K. M. & Freeman, G. J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. (2020). [Epub ahead of print].
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Zhao, S. et al. Toll-like receptors and prostate cancer. Front Immunol. 5, 352 (2014).
Zhang, D. et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004).
Yarovinsky, F. et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629 (2005).
Nishiya, T. et al. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J. Biol. Chem. 280, 37107–37117 (2005).
Carpentier, A. et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol. 12, 401–408 (2010).
Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5, 190–198 (2004).
Liu, Y. et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 30, 243–256 (2016).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 (2013).
Coffelt, S. B. et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Wu, C. F. et al. The lack of type I interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int J. Cancer 137, 837–847 (2015).
Liu, Y. & Cao, X. Immunosuppressive cells in tumor immune escape and metastasis. J. Mol. Med (Berl.) 94, 509–522 (2016).
Zhang, X. et al. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol. Cancer 17, 146 (2018).
Giri, P. K. & Schorey, J. S. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS ONE 3, e2461 (2008).
Schorey, J. S. et al. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 16, 24–43 (2015).
Mantel, P. Y. et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13, 521–534 (2013).
Zhang, Y. et al. Extracellular vesicles derived from ODN-stimulated macrophages transfer and activate Cdc42 in recipient cells and thereby increase cellular permissiveness to EV uptake. Sci. Adv. 5, eaav1564 (2019).
Ishii, N. et al. Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells. J. Immunol. 193, 5118–5128 (2014).
Fabbri, M. et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl Acad. Sci. USA 109, E2110–E2116 (2012).
Liu, Y. et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am. J. Pathol. 176, 2490–2499 (2010).
Chalmin, F. et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest 120, 457–471 (2010).
Li, X. et al. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFkappaB-TLR signaling pathway. J. Hematol. Oncol. 9, 42 (2016).
Chow, A. et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci. Rep. 4, 5750 (2014).
Bretz, N. P. et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J. Biol. Chem. 288, 36691–36702 (2013).
Im, H. et al. Novel nanosensing technologies for exosome detection and profiling. Lab Chip 17, 2892–2898 (2017).
Witwer, K. W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360 (2013).
Mateescu, B. et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J. Extracell. Vesicles 6, 1286095 (2017).
Momen-Heravi, F. et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 394, 1253–1262 (2013).
Li, P. et al. Progress in exosome isolation techniques. Theranostics 7, 789–804 (2017).
Siravegna, G. et al. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 14, 531–548 (2017).
Nolte-‘T Hoen, E. N. et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic. Acids Res. 40, 9272–9285 (2012).
Li, Y. et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 25, 981–984 (2015).
Dou, Y. et al. Circular RNAs are down-regulated in KRAS mutant colon cancer cells and can be transferred to exosomes. Sci. Rep. 6, 37982 (2016).
Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182 (2015).
Moutinho-Ribeiro, P., Melo, S. & Macedo, G. Glypican-1 circulating exosomes: a promising clue to individualize surveillance of pancreatic cysts?. Eur. Radio. 28, 3018–3019 (2018).
Diamandis, E. P. & Plebani, M. Glypican-1 as a highly sensitive and specific pancreatic cancer biomarker. Clin. Chem. Lab Med 54, e1–e2 (2016).
Frampton, A. E. et al. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget 9, 19006–19013 (2018).
Xiao, D. et al. Combined exosomal GPC1, CD82, and serum CA19-9 as multiplex targets: a specific, sensitive, and reproducible detection panel for the diagnosis of pancreatic cancer. Mol. Cancer Res. 18, 300–310 (2019).
Alegre, E. et al. Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin. Chim. Acta 454, 28–32 (2016).
Kimura, H. et al. CKAP4, a DKK1 receptor, is a biomarker in exosomes derived from pancreatic cancer and a molecular target for therapy. Clin. Cancer Res 25, 1936–1947 (2019).
Wang, J., Zheng, Y. & Zhao, M. Exosome-based cancer therapy: implication for targeting cancer stem cells. Front Pharm. 7, 533 (2016).
Ha, D., Yang, N. & Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B 6, 287–296 (2016).
Pi, F. et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 13, 82–89 (2018).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Viaud, S. et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS ONE 4, e4942 (2009).
Dai, S. et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 16, 782–790 (2008).
Escudier, B. et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med 3, 10 (2005).
Morse, M. A. et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med 3, 9 (2005).
Shimasaki, N. et al. Expanded and armed natural killer cells for cancer treatment. Cytotherapy 18, 1422–1434 (2016).
Zhu, L. et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics 7, 2732–2745 (2017).
Cheng, L., Wang, Y. & Huang, L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol. Ther. 25, 1665–1675 (2017).
Andre, F. et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol. 172, 2126–2136 (2004).
Besse, B. et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5, e1071008 (2016).
Schreiner, B. et al. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J. Neuroimmunol. 155, 172–182 (2004).
Tian, H. & Li, W. Dendritic cell-derived exosomes for cancer immunotherapy: hope and challenges. Ann. Transl. Med. 5, 221 (2017).
Pitt, J. M. et al. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Invest 126, 1224–1232 (2016).
Hargadon, K. M. et al. Melanoma-derived factors alter the maturation and activation of differentiated tissue-resident dendritic cells. Immunol. Cell Biol. 94, 24–38 (2016).
Krempski, J. et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J. Immunol. 186, 6905–6913 (2011).
Karyampudi, L. et al. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 74, 2974–2985 (2014).
Kenkel, J. A. et al. An immunosuppressive dendritic cell subset accumulates at secondary sites and promotes metastasis in pancreatic cancer. Cancer Res 77, 4158–4170 (2017).
D, H. Y. & Appel, S. Current status and future perspectives of dendritic cell-based cancer immunotherapy. Scand. J. Immunol. 78, 167–171 (2013).
Sabado, R. L. & Bhardwaj, N. Directing dendritic cell immunotherapy towards successful cancer treatment. Immunotherapy 2, 37–56 (2010).
Temchura, V. V. et al. Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus. Vaccine 26, 3662–3672 (2008).
Robbins, P. D., Dorronsoro, A. & Booker, C. N. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Invest 126, 1173–1180 (2016).
Pitt, J. M., Kroemer, G. & Zitvogel, L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J. Clin. Invest 126, 1139–1143 (2016).
Schorey, J. S. & Harding, C. V. Extracellular vesicles and infectious diseases: new complexity to an old story. J. Clin. Invest 126, 1181–1189 (2016).
Lindenbergh, M. F. S. & Stoorvogel, W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu Rev. Immunol. 36, 435–459 (2018).
Czernek, L. & Duchler, M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch. Immunol. Ther. Exp. (Warsz.) 65, 311–323 (2017).
Bach, D. H. et al. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int J. Cancer 141, 220–230 (2017).
Van Niel, G., D’angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Fleissner, F., Bonn, M. & Parekh, S. H. Microscale spatial heterogeneity of protein structural transitions in fibrin matrices. Sci. Adv. 2, e1501778 (2016).
Chang, J. Y. Structural heterogeneity of 6 M GdmCl-denatured proteins: implications for the mechanism of protein folding. Biochemistry 48, 9340–9346 (2009).
Keedy, D. A. Conformational and connotational heterogeneity: a surprising relationship between protein structural flexibility and puns. Proteins 83, 797–798 (2015).
Antonyak, M. A., Lukey, M. J. & Cerione, R. A. Lipid-filled vesicles modulate macrophages. Science 363, 931–932 (2019).
Kalluri, R. & Lebleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
Veerman, R. E. et al. Immune cell-derived extracellular vesicles - functions and therapeutic applications. Trends Mol. Med. 25, 382–394 (2019).
Tan, A., De La Pena, H. & Seifalian, A. M. The application of exosomes as a nanoscale cancer vaccine. Int J. Nanomed. 5, 889–900 (2010).
Acknowledgements
We would like to apologize to those researchers whose related work we were not able to cite in this review. This work was supported by a special program from the Chinese National Natural Science Funds (31701232 to F.X.; 31671457 and 91753139 to L.Z.; and 31871405 and 31571460 to F.Z.), the National Postdoctoral Program for Innovative Talents (BX201700165 to F.X.), the National Science Foundation for Postdoctoral Scientists of China (BX201700165 to F.X.), Distinguished Young Scholars of Jiangsu Province (BK20180043 to F.Z.), the Key Project of University Natural Science Foundation of Jiangsu Province (19KJA550003 to F.Z.), the Shenzhen Basic Research Program (JCYJ20180507182203049 to S.Z.) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
X.Z. and F.X. conceived and drafted the paper. X.Z., L.W., F.X., and F.Z. discussed the concepts presented in the paper. X.Z. generated the figures. F.Z. approved the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhou, X., Xie, F., Wang, L. et al. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol Immunol 17, 323–334 (2020). https://doi.org/10.1038/s41423-020-0391-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-020-0391-1
Keywords
This article is cited by
-
Exosomal secreted SCIMP regulates communication between macrophages and neutrophils in pneumonia
Nature Communications (2024)
-
Extracellular vesicles as tools and targets in therapy for diseases
Signal Transduction and Targeted Therapy (2024)
-
Global trend in exosome isolation and application: an update concept in management of diseases
Molecular and Cellular Biochemistry (2024)
-
Adipose-derived extracellular vesicles – a novel cross-talk mechanism in insulin resistance, non-alcoholic fatty liver disease, and polycystic ovary syndrome
Endocrine (2024)
-
Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME
Journal of Biomedical Science (2023)