Abstract
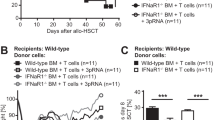
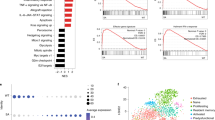
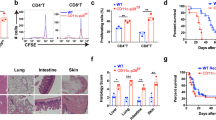
Stimulator of interferon genes (STING)-mediated innate immune activation plays a key role in tumor- and self-DNA-elicited antitumor immunity and autoimmunity. However, STING can also suppress tumor immunity and autoimmunity. STING signaling in host nonhematopoietic cells was reported to either protect against or promote graft-versus-host disease (GVHD), a major complication of allogeneic hematopoietic cell transplantation (allo-HCT). Host hematopoietic antigen-presenting cells (APCs) play key roles in donor T-cell priming during GVHD initiation. However, how STING regulates host hematopoietic APCs after allo-HCT remains unknown. We utilized murine models of allo-HCT to assess the role of STING in hematopoietic APCs. STING-deficient recipients developed more severe GVHD after major histocompatibility complex-mismatched allo-HCT. Using bone marrow chimeras, we found that STING deficiency in host hematopoietic cells was primarily responsible for exacerbating the disease. Furthermore, STING on host CD11c+ cells played a dominant role in suppressing allogeneic T-cell responses. Mechanistically, STING deficiency resulted in increased survival, activation, and function of APCs, including macrophages and dendritic cells. Consistently, constitutive activation of STING attenuated the survival, activation, and function of APCs isolated from STING V154M knock-in mice. STING-deficient APCs augmented donor T-cell expansion, chemokine receptor expression, and migration into intestinal tissues, resulting in accelerated/exacerbated GVHD. Using pharmacologic approaches, we demonstrated that systemic administration of a STING agonist (bis-(3′-5′)-cyclic dimeric guanosine monophosphate) to recipient mice before transplantation significantly reduced GVHD mortality. In conclusion, we revealed a novel role of STING in APC activity that dictates T-cell allogeneic responses and validated STING as a potential therapeutic target for controlling GVHD after allo-HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Motwani, M., Pesiridis, S. & Fitzgerald, K. A. DNA sensing by the cGAS-STING pathway in health and disease. Nat. Rev. Genet. 20, 657–674 (2019).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Ahn, J., Gutman, D., Saijo, S. & Barber, G. N. STING manifests self DNA-dependent inflammatory disease. Proc. Natl Acad. Sci. 109, 19386–19391 (2012).
Woo, S.-R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Kwon, J. & Bakhoum, S. F. The cytosolic DNA-sensing cGAS–STING pathway in cancer. Cancer Discov. 10, 26–39 (2020).
Perkey, E. & Maillard, I. New Insights into graft-versus-host disease and graft rejection. Annu. Rev. Pathol. 13, 219–245 (2018).
Zeiser, R. & Blazar, B. R. Acute graft-versus-host disease—biologic process, prevention, and therapy. N. Engl. J. Med. 377, 2167–2179 (2017).
Bader, C. S. et al. STING differentially regulates experimental GVHD mediated by CD8 versus CD4 T cell subsets. Sci. Transl. Med. 12 https://doi.org/10.1126/scitranslmed.aay5006 (2020).
Fischer, J. C. et al. RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci. Transl. Med. 9 https://doi.org/10.1126/scitranslmed.aag2513 (2017).
Koyama, M. & Hill, G. R. Alloantigen presentation and graft-versus-host disease: fuel for the fire. Blood 127, 2963–2970 (2016).
Tang, C. A. et al. STING regulates BCR signaling in normal and malignant B cells. Cell. Mol. Immunol. https://doi.org/10.1038/s41423-020-00552-0 (2020).
Fredricks, D. N. The gut microbiota and graft-versus-host disease. J. Clin. Investig. 129, 1808–1817 (2019).
Cerboni, S. et al. Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes. J. Exp. Med. 214, 1769–1785 (2017).
Gulen, M. F. et al. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 8, 1–10 (2017).
Koyama, M. et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat. Med. 18, 135–142 (2011).
Koyama, M. et al. MHC class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity 51, 885–898.e887 (2019).
Zhu, Y. et al. STING: a master regulator in the cancer-immunity cycle. Mol. Cancer 18, 152 (2019).
Hume, D. A. Macrophages as APC and the dendritic cell myth. J. Immunol. 181, 5829–5835 (2008).
Arnold, I. C. et al. CD11c+ monocyte/macrophages promote chronic Helicobacter hepaticus-induced intestinal inflammation through the production of IL-23. Mucosal Immunol. 9, 352–363 (2016).
Koyama, M. & Hill, G. R. The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease. Blood 134, 2139–2148 (2019).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Zhang, Y. et al. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J. Immunol. 169, 7111–7118 (2002).
Schwab, L. et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat. Med. 20, 648–654 (2014).
Garcia, J. A. et al. Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J. Immunol. 191, 1063–1072 (2013).
Wysocki, C. A., Panoskaltsis-Mortari, A., Blazar, B. R. & Serody, J. S. Leukocyte migration and graft-versus-host disease. Blood 105, 4191–4199 (2005).
Zhou, V. et al. A colitogenic memory CD4+ T cell population mediates gastrointestinal graft-versus-host disease. J. Clin. Investig. 126, 3541–3555 (2016).
Burdette, D. L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011).
Karaolis, D. K. et al. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol. 178, 2171–2181 (2007).
Gall, A. et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36, 120–131 (2012).
Larkin, B. et al. Cutting edge: activation of STING in T cells induces type I IFN responses and cell death. J. Immunol. 199, 397–402 (2017).
Liang, D. et al. Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochim. Biophys. Acta 1852, 2494–2503 (2015).
Lemos, H. et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 76, 2076–2081 (2016).
Sharma, S. et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc. Natl Acad. Sci. 112, E710–E717 (2015).
Gaidt, M. M. et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell 171, 1110–1124.e1118 (2017).
Jeremiah, N. et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Investig. 124, 5516–5520 (2014).
Warner, J. D. et al. STING-associated vasculopathy develops independently of IRF3 in mice. J. Exp. Med. 214, 3279–3292 (2017).
Bennion, B. G. et al. A human gain-of-function STING mutation causes immunodeficiency and gammaherpesvirus-induced pulmonary fibrosis in mice. J. Virol. 93, e01806-18 (2019).
Crow, M. K., Olferiev, M., Kirou, K. A. & Type, I. Interferons in autoimmune disease. Annu. Rev. Pathol. 14, 369–393 (2019).
Kotredes, K. P., Thomas, B. & Gamero, A. M. The protective role of type I interferons in the gastrointestinal tract. Front. Immunol. 8, 410 (2017).
Petrasek, J. et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl Acad. Sci. USA 110, 16544–16549 (2013).
Tang, C.-H. A. et al. Agonist-mediated activation of STING induces apoptosis in malignant B cells. Cancer Res. 76, 2137–2152 (2016).
Huang, L. et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J. Immunol. 191, 3509–3513 (2013).
Jasperson, L. K. et al. Inducing the tryptophan catabolic pathway, indoleamine 2, 3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood 114, 5062–5070 (2009).
Galleu, A. et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 9 https://doi.org/10.1126/scitranslmed.aam7828 (2017).
Koyama, M. et al. MHC class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity 51, 885–898. e887 (2019).
Hashimoto, D. et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J. Exp. Med. 208, 1069–1082 (2011).
Duffner, U. A. et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 172, 7393–7398 (2004).
Weber, M. et al. Host-derived CD8(+) dendritic cells protect against acute graft-versus-host disease after experimental allogeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 20, 1696–1704 (2014).
Hadeiba, H. et al. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat. Immunol. 9, 1253–1260 (2008).
Acknowledgements
We appreciate the technical support provided by the Department of Lab Animal Research (DLAR) and the Flow Cytometry Core at MUSC. This work was supported in part by the Hollings Cancer Center Fellowship (to Y.W.), NIH Grant R01CA163910 (to C.-C.A.H.), and NIH R01s AI118305, HL137373, and HL140953 (to X.-Z.Y.).
Author information
Authors and Affiliations
Contributions
Y.W. designed and performed the research, collected, analyzed, and interpreted the data, and drafted and revised the paper; C.-H.A.T. generated the STINGflox and STING V154M mice; C.M. performed the research, collected and analyzed the data, and edited the paper; D.B., M.H.S., L.T., S.S., H.-J.C., T.T., and M.Z. assisted in collecting data and editing the paper; L.H. and A.L.M. interpreted the data and edited the paper; and C.-C.A.H. and X.-Z.Y. designed the research, interpreted the data, and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, Y., Tang, CH.A., Mealer, C. et al. STING negatively regulates allogeneic T-cell responses by constraining antigen-presenting cell function. Cell Mol Immunol 18, 632–643 (2021). https://doi.org/10.1038/s41423-020-00611-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-020-00611-6