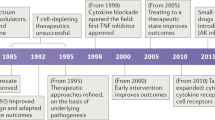
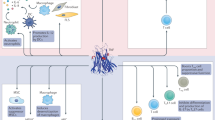
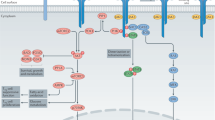
Abstract
In the last decade, approaches based on T cells and their immunomodulatory receptors have emerged as a solid improvement in treatments for various types of cancer. However, the roles of these molecules in the therapeutic context of autoimmune and cardiovascular diseases are still relatively unexplored. Here, we review the best known and most commonly used immunomodulatory T cell receptors in clinical practice (PD-1 and CTLA-4), along with the rest of the receptors with known functions in animal models, which have great potential as modulators in human pathologies in the medium term. Among these other receptors is the receptor CD69, which has recently been described to be expressed in mouse and human T cells in autoimmune and cardiovascular diseases and cancer. However, inhibition of these receptors individually or in combination by drugs or monoclonal antibodies generates a loss of immunological tolerance and can trigger multiple autoimmune disorders in different organs and immune-related adverse effects. In the coming decades, knowledge on the functions of different immunomodulatory receptors will be pivotal for the development of new and better therapies with less harmful side effects. In this review, we discuss the roles of these receptors in the control of immunity from a perspective focused on therapeutic potential in not only cancer but also autoimmune diseases, such as systemic lupus erythematosus, autoimmune diabetes and rheumatoid arthritis, and cardiovascular diseases, such as atherosclerosis, acute myocardial infarction, and myocarditis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boardman, D. A. & Levings, M. K. Cancer immunotherapies repurposed for use in autoimmunity. Nat. Biomed. Eng. 3, 259–263 (2019).
Lutgens, E. et al. Immunotherapy for cardiovascular disease. Eur. Heart J. 40, 3937–3946 (2019).
Murphy, K. A. et al. Immunomodulatory receptors are differentially expressed in B and T cell subsets relevant to autoimmune disease. Clin. Immunol. 209, 108276 (2019).
Gonzalez-Amaro, R., Cortes, J. R., Sanchez-Madrid, F. & Martin, P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol. Med. 19, 625–632 (2013).
Watanabe, N. et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 4, 670–679 (2003).
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2018).
Krummel, M. F. & Allison, J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Liu, X. et al. Cutting edge: a critical role of B and T lymphocyte attenuator in peripheral T cell tolerance induction. J. Immunol. 182, 4516–4520 (2009).
Sedy, J. R. et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 6, 90–98 (2005).
Huard, B., Prigent, P., Tournier, M., Bruniquel, D. & Triebel, F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 25, 2718–2721 (1995).
Anderson, A. C., Joller, N. & Kuchroo, V. K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016).
Zhu, C. et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6, 1245–1252 (2005).
Huang, Y. H. et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517, 386–390 (2015).
Harjunpaa, H. & Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 200, 108–119 (2020).
Reches, A. et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J. Immunother. Cancer 8, https://doi.org/10.1136/jitc-2019-000266 (2020).
Yu, X. et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 10, 48–57 (2009).
Cannons, J. L., Tangye, S. G. & Schwartzberg, P. L. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 29, 665–705 (2011).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37 (2009).
Nowak, E. C. et al. Immunoregulatory functions of VISTA. Immunol. Rev. 276, 66–79 (2017).
Wang, L. et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 208, 577–592 (2011).
Jones, N. H. et al. Isolation of complementary DNA clones encoding the human lymphocyte glycoprotein T1/Leu-1. Nature 323, 346–349 (1986).
Huang, H. J., Jones, N. H., Strominger, J. L. & Herzenberg, L. A. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: molecular homology to its human counterpart Leu-1/T1 (CD5). Proc. Natl Acad. Sci. USA 84, 204–208 (1987).
Brossard, C., Semichon, M., Trautmann, A. & Bismuth, G. CD5 inhibits signaling at the immunological synapse without impairing its formation. J. Immunol. 170, 4623–4629 (2003).
Tabbekh, M., Mokrani-Hammani, M., Bismuth, G. & Mami-Chouaib, F. T-cell modulatory properties of CD5 and its role in antitumor immune responses. Oncoimmunology 2, e22841 (2013).
Sancho, D., Gomez, M. & Sanchez-Madrid, F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 26, 136–140 (2005).
Lopez-Cabrera, M. et al. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J. Exp. Med. 178, 537–547 (1993).
Martin, P. & Sanchez-Madrid, F. CD69: an unexpected regulator of TH17 cell-driven inflammatory responses. Sci. Signal. 4, pe14 (2011).
Martin, P. et al. CD69 association with Jak3/Stat5 proteins regulates Th17 cell differentiation. Mol. Cell Biol. 30, 4877–4889 (2010).
de la Fuente, H. et al. The leukocyte activation receptor CD69 controls T cell differentiation through its interaction with galectin-1. Mol. Cell Biol. 34, 2479–2487 (2014).
Lin, C. R. et al. Glycosylation-dependent interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell differentiation. FASEB J. 29, 5006–5017 (2015).
Tsilingiri, K. et al. Oxidized low-density lipoprotein receptor in lymphocytes prevents atherosclerosis and predicts subclinical disease. Circulation 139, 243–255 (2019).
Cibrian, D. et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 17, 985–996 (2016).
Rosenblum, M. D., Remedios, K. A. & Abbas, A. K. Mechanisms of human autoimmunity. J. Clin. Invest. 125, 2228–2233 (2015).
Nishimura, H., Honjo, T. & Minato, N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J. Exp. Med. 191, 891–898 (2000).
Sharpe, A. H., Wherry, E. J., Ahmed, R. & Freeman, G. J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8, 239–245 (2007).
Nishimura, H., Nose, M., Hiai, H., Minato, N. & Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999).
Kasagi, S. et al. Anti-programmed cell death 1 antibody reduces CD4+PD-1+ T cells and relieves the lupus-like nephritis of NZB/W F1 mice. J. Immunol. 184, 2337–2347 (2010).
Wong, M., La Cava, A., Singh, R. P. & Hahn, B. H. Blockade of programmed death-1 in young (New Zealand black x New Zealand white)F1 mice promotes the activity of suppressive CD8+ T cells that protect from lupus-like disease. J. Immunol. 185, 6563–6571 (2010).
Salama, A. D. et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 198, 71–78 (2003).
Ansari, M. J. et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198, 63–69 (2003).
Sage, P. T., Francisco, L. M., Carman, C. V. & Sharpe, A. H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 14, 152–161 (2013).
Kristjansdottir, H. et al. Lower expression levels of the programmed death 1 receptor on CD4+CD25+ T cells and correlation with the PD-1.3A genotype in patients with systemic lupus erythematosus. Arthritis Rheum. 62, 1702–1711 (2010).
Bertsias, G. K. et al. Genetic, immunologic, and immunohistochemical analysis of the programmed death 1/programmed death ligand 1 pathway in human systemic lupus erythematosus. Arthritis Rheum. 60, 207–218 (2009).
Shi, H. et al. Elevated serum autoantibodies against co-inhibitory PD-1 facilitate T cell proliferation and correlate with disease activity in new-onset systemic lupus erythematosus patients. Arthritis Res. Ther. 19, 52 (2017).
Jiao, Q. et al. Upregulated PD-1 expression is associated with the development of systemic lupus erythematosus, but not the PD-1.1 allele of the PDCD1 gene. Int J. Genomics 2014, 950903 (2014).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Stohl, W. et al. Global T cell dysregulation in non-autoimmune-prone mice promotes rapid development of BAFF-independent, systemic lupus erythematosus-like autoimmunity. J. Immunol. 181, 833–841 (2008).
Barreto, M. et al. Evidence for CTLA4 as a susceptibility gene for systemic lupus erythematosus. Eur. J. Hum. Genet. 12, 620–626 (2004).
Ahmed, S. et al. Association of CTLA-4 but not CD28 gene polymorphisms with systemic lupus erythematosus in the Japanese population. Rheumatology 40, 662–667 (2001).
Hudson, L. L., Rocca, K., Song, Y. W. & Pandey, J. P. CTLA-4 gene polymorphisms in systemic lupus erythematosus: a highly significant association with a determinant in the promoter region. Hum. Genet. 111, 452–455 (2002).
Jury, E. C. et al. Abnormal CTLA-4 function in T cells from patients with systemic lupus erythematosus. Eur. J. Immunol. 40, 569–578 (2010).
Dahal, L. N. et al. Immunoregulatory soluble CTLA-4 modifies effector T-cell responses in systemic lupus erythematosus. Arthritis Res. Ther. 18, 180 (2016).
Merrill, J. T. et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 62, 3077–3087 (2010).
Furie, R. et al. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol. 66, 379–389 (2014).
Oster, C. et al. BTLA expression on Th1, Th2 and Th17 effector T-cells of patients with systemic lupus erythematosus is associated with active disease. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20184505 (2019).
Sawaf, M. et al. Defective BTLA functionality is rescued by restoring lipid metabolism in lupus CD4+ T cells. JCI Insight 3, https://doi.org/10.1172/jci.insight.99711 (2018).
Vendel, A. C. et al. B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J. Immunol. 182, 1509–1517 (2009).
Otsuki, N., Kamimura, Y., Hashiguchi, M. & Azuma, M. Expression and function of the B and T lymphocyte attenuator (BTLA/CD272) on human T cells. Biochem. Biophys. Res. Commun. 344, 1121–1127 (2006).
Lauzurica, P. et al. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood 95, 2312–2320 (2000).
Sanchez-Diaz, R. et al. Thymus-derived regulatory T cell development is regulated by C-type lectin-mediated BIC/MicroRNA 155 expression. Mol. Cell. Biol. 37, https://doi.org/10.1128/MCB.00341-16 (2017).
Cortes, J. R. et al. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. J. Autoimmun. 55, 51–62 (2014).
Radulovic, K. et al. CD69 regulates type I IFN-induced tolerogenic signals to mucosal CD4 T cells that attenuate their colitogenic potential. J. Immunol. 188, 2001–2013 (2012).
Yu, L. et al. CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production. Cell Death Dis. 9, 905 (2018).
Ishikawa, S. et al. A subset of CD4+ T cells expressing early activation antigen CD69 in murine lupus: possible abnormal regulatory role for cytokine imbalance. J. Immunol. 161, 1267–1273 (1998).
Peixoto, T. V. et al. CD4(+)CD69(+) T cells and CD4(+)CD25(+)FoxP3(+) Treg cells imbalance in peripheral blood, spleen and peritoneal lavage from pristane-induced systemic lupus erythematosus (SLE) mice. Adv. Rheumatol. 59, 30 (2019).
Su, C. C., Shau, W. Y., Wang, C. R., Chuang, C. Y. & Chen, C. Y. CD69 to CD3 ratio of peripheral blood mononuclear cells as a marker to monitor systemic lupus erythematosus disease activity. Lupus 6, 449–454 (1997).
Portales-Perez, D., Gonzalez-Amaro, R., Abud-Mendoza, C. & Sanchez-Armass, S. Abnormalities in CD69 expression, cytosolic pH and Ca2+ during activation of lymphocytes from patients with systemic lupus erythematosus. Lupus 6, 48–56 (1997).
Vitales-Noyola, M. et al. Patients with systemic lupus erythematosus show increased levels and defective function of CD69(+) T regulatory cells. Mediators Inflamm. 2017, 2513829 (2017).
Han, Y., Guo, Q., Zhang, M., Chen, Z. & Cao, X. CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J. Immunol. 182, 111–120 (2009).
Crispin, J. C., Martinez, A., de Pablo, P., Velasquillo, C. & Alcocer-Varela, J. Participation of the CD69 antigen in the T-cell activation process of patients with systemic lupus erythematosus. Scand. J. Immunol. 48, 196–200 (1998).
Vitales-Noyola, M. et al. Quantitative and functional analysis of CD69(+) NKG2D(+) T regulatory cells in healthy subjects. Hum. Immunol. 76, 511–518 (2015).
Rodriguez-Munoz, A. et al. Levels of regulatory T cells CD69(+)NKG2D(+)IL-10(+) are increased in patients with autoimmune thyroid disorders. Endocrine 51, 478–489 (2016).
Vitales-Noyola, M. et al. Quantitative and functional analysis of CD69(+) T regulatory lymphocytes in patients with periodontal disease. J. Oral. Pathol. Med. 46, 549–557 (2017).
Tsujimura, S., Adachi, T., Saito, K. & Tanaka, Y. Role of P-glycoprotein on CD69(+)CD4(+) cells in the pathogenesis of proliferative lupus nephritis and non-responsiveness to immunosuppressive therapy. RMD Open 3, e000423 (2017).
El Khatib, M. M. et al. beta-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection. Gene Ther. 22, 430–438 (2015).
Li, R. et al. PD-L1-driven tolerance protects neurogenin3-induced islet neogenesis to reverse established type 1 diabetes in NOD mice. Diabetes 64, 529–540 (2015).
Sun, P. et al. Unlike PD-L1, PD-1 is downregulated on partial immune cells in type 2 diabetes. J. Diabetes Res. 2019, 5035261 (2019).
Colli, M. L. et al. PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-alpha and-gamma via IRF1 induction. EBioMedicine 36, 367–375 (2018).
Osum, K. C. et al. Interferon-gamma drives programmed death-ligand 1 expression on islet beta cells to limit T cell function during autoimmune diabetes. Sci. Rep. 8, 8295 (2018).
Lenschow, D. J. et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 181, 1145–1155 (1995).
Truong, W. et al. BTLA targeting modulates lymphocyte phenotype, function, and numbers and attenuates disease in nonobese diabetic mice. J. Leukoc. Biol. 86, 41–51 (2009).
Bekiaris, V., Sedy, J. R., Macauley, M. G., Rhode-Kurnow, A. & Ware, C. F. The inhibitory receptor BTLA controls gammadelta T cell homeostasis and inflammatory responses. Immunity 39, 1082–1094 (2013).
Bettini, M. et al. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. J. Immunol. 187, 3493–3498 (2011).
Okazaki, T. et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 208, 395–407 (2011).
Zhang, Q. et al. LAG3 limits regulatory T cell proliferation and function in autoimmune diabetes. Sci. Immunol. 2, https://doi.org/10.1126/sciimmunol.aah4569 (2017).
Fuhrman, C. A. et al. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J. Immunol. 195, 145–155 (2015).
Diarte-Anazco, E. M. G. et al. Novel insights into the role of HDL-associated sphingosine-1-phosphate in cardiometabolic diseases. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20246273 (2019).
Srinivasan, S. et al. Sphingosine-1-phosphate reduces CD4+ T-cell activation in type 1 diabetes through regulation of hypoxia-inducible factor short isoform I.1 and CD69. Diabetes 57, 484–493 (2008).
Klocke, K., Sakaguchi, S., Holmdahl, R. & Wing, K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl Acad. Sci. USA 113, E2383–E2392 (2016).
Verma, N., Burns, S. O., Walker, L. S. K. & Sansom, D. M. Immune deficiency and autoimmunity in patients with CTLA-4 (CD152) mutations. Clin. Exp. Immunol. 190, 1–7 (2017).
Kuehn, H. S. et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 345, 1623–1627 (2014).
Lin, J. et al. TNFalpha blockade in human diseases: an overview of efficacy and safety. Clin. Immunol. 126, 13–30 (2008).
Genant, H. K. et al. Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial. Ann. Rheum. Dis. 67, 1084–1089 (2008).
Ruperto, N. et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet 372, 383–391 (2008).
Larsen, C. P. et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am. J. Transpl. 5, 443–453 (2005).
Oki, M. et al. A functional polymorphism in B and T lymphocyte attenuator is associated with susceptibility to rheumatoid arthritis. Clin. Dev. Immunol. 2011, 305656 (2011).
Yang, B. et al. The expression of BTLA was increased and the expression of HVEM and LIGHT were decreased in the T cells of patients with rheumatoid arthritis [corrected]. PLoS ONE 11, e0155345 (2016).
Nakachi, S. et al. Interleukin-10-producing LAG3(+) regulatory T cells are associated with disease activity and abatacept treatment in rheumatoid arthritis. Arthritis Res. Ther. 19, 97 (2017).
Chen, S. Y., Hsu, W. T., Chen, Y. L., Chien, C. H. & Chiang, B. L. Lymphocyte-activation gene 3(+) (LAG3(+)) forkhead box protein 3(-) (FOXP3(-)) regulatory T cells induced by B cells alleviates joint inflammation in collagen-induced arthritis. J. Autoimmun. 68, 75–85 (2016).
Lee, J. et al. Expression of human TIM-3 and its correlation with disease activity in rheumatoid arthritis. Scand. J. Rheumatol. 40, 334–340 (2011).
Koohini, Z. et al. Analysis of PD-1 and Tim-3 expression on CD4(+) T cells of patients with rheumatoid arthritis; negative association with DAS28. Clin. Rheumatol. 37, 2063–2071 (2018).
Zhao, W. et al. TIGIT overexpression diminishes the function of CD4 T cells and ameliorates the severity of rheumatoid arthritis in mouse models. Exp. Cell Res. 340, 132–138 (2016).
Fasth, A. E., Bjorkstrom, N. K., Anthoni, M., Malmberg, K. J. & Malmstrom, V. Activating NK-cell receptors co-stimulate CD4(+)CD28(−) T cells in patients with rheumatoid arthritis. Eur. J. Immunol. 40, 378–387 (2010).
Ceeraz, S. et al. VISTA deficiency attenuates antibody-induced arthritis and alters macrophage gene expression in response to simulated immune complexes. Arthritis Res. Ther. 19, 270 (2017).
Verwilghen, J., Corrigall, V., Pope, R. M., Rodrigues, R. & Panayi, G. S. Expression and function of CD5 and CD28 in patients with rheumatoid arthritis. Immunology 80, 96–102 (1993).
Martin, P. et al. The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity. J. Allergy Clin. Immunol. 126, 355–365, 365 e351–e353 (2010).
Cruz-Adalia, A. et al. CD69 limits the severity of cardiomyopathy after autoimmune myocarditis. Circulation 122, 1396–1404 (2010).
Sancho, D. et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J. Clin. Invest. 112, 872–882 (2003).
Hernandez-Garcia, C., Fernandez-Gutierrez, B., Morado, I. C., Banares, A. A. & Jover, J. A. The CD69 activation pathway in rheumatoid arthritis synovial fluid T cells. Arthritis Rheum. 39, 1277–1286 (1996).
Iannone, F., Corrigal, V. M. & Panayi, G. S. CD69 on synovial T cells in rheumatoid arthritis correlates with disease activity. Br. J. Rheumatol. 35, 397 (1996).
Steinbach, K., Vincenti, I., Merkler, D. & Resident-Memory, T. Cells in tissue-restricted immune responses: for better or worse? Front. Immunol. 9, 2827 (2018).
Blanton, R. M., Carrillo-Salinas, F. J. & Alcaide, P. T-cell recruitment to the heart: friendly guests or unwelcome visitors? Am. J. Physiol. Heart Circ. Physiol. 317, H124–H140 (2019).
Munro, J. M., van der Walt, J. D., Munro, C. S., Chalmers, J. A. & Cox, E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum. Pathol. 18, 375–380 (1987).
Ridker, P. M. et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N. Engl. J. Med 377, 1119–1131 (2017).
Palinski, W. Immunomodulation: a new role for statins? Nat. Med. 6, 1311–1312 (2000).
Mach, F. Statins as immunomodulatory agents. Circulation 109, II15–II17 (2004).
Tse, K., Tse, H., Sidney, J., Sette, A. & Ley, K. T cells in atherosclerosis. Int Immunol. 25, 615–622 (2013).
van Duijn, J., Kuiper, J. & Slutter, B. The many faces of CD8+ T cells in atherosclerosis. Curr. Opin. Lipido. 29, 411–416 (2018).
Qiu, M. K. et al. PD-1 and Tim-3 pathways regulate CD8+ T cells function in atherosclerosis. PLoS ONE 10, e0128523 (2015).
Li, S. H., Chen, W. J., Yan, M., Shu, Y. W. & Liao, Y. H. Expression of coinhibitory PD-L1 on CD4(+)CD25(+)FOXP3(+) regulatory T cells is elevated in patients with acute coronary syndrome. Coron. Artery Dis. 26, 598–603 (2015).
Lee, J. et al. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J. Mol. Cell Cardiol. 46, 169–176 (2009).
Foks, A. C. et al. T-cell immunoglobulin and mucin domain 3 acts as a negative regulator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 33, 2558–2565 (2013).
Ma, K. et al. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE(−/−) mice. Cardiovasc. Res. 97, 349–359 (2013).
Ewing, M. M. et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int. J. Cardiol. 168, 1965–1974 (2013).
Matsumoto, T. et al. Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 36, 1141–1151 (2016).
Golden, D. et al. Lymphocyte activation gene 3 and coronary artery disease. JCI Insight 1, e88628 (2016).
Douna, H. et al. B- and T-lymphocyte attenuator stimulation protects against atherosclerosis by regulating follicular B cells. Cardiovasc. Res. 116, 295–305 (2020).
Foks, A. C. et al. Agonistic anti-TIGIT treatment inhibits T cell responses in LDLr deficient mice without affecting atherosclerotic lesion development. PLoS ONE 8, e83134 (2013).
Gao, J. et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 23, 551–555 (2017).
Decano, J. L. & Aikawa, M. Dynamic macrophages: understanding mechanisms of activation as guide to therapy for atherosclerotic vascular disease. Front. Cardiovasc. Med. 5, 97 (2018).
Roselaar, S. E., Kakkanathu, P. X. & Daugherty, A. Lymphocyte populations in atherosclerotic lesions of apoE −/− and LDL receptor −/− mice. Decreasing density with disease progression. Arterioscler. Thromb. Vasc. Biol. 16, 1013–1018 (1996).
Sekiya, T. et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 14, 230–237 (2013).
Lim, H. et al. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 40, 153–165 (2014).
Fernandez-Friera, L. et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 131, 2104–2113 (2015).
Swirski, F. K. & Nahrendorf, M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 18, 733–744 (2018).
Lutgens, E. & Seijkens, T. T. P. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J. Immunother. Cancer 8, https://doi.org/10.1136/jitc-2019-000300 (2020).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 6, 38 (2020).
Zaha, V. G., Meijers, W. C. & Moslehi, J. Cardio-immuno-oncology. Circulation 141, 87–89 (2020).
Bar, J. et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur. J. Cancer 120, 122–131 (2019).
Newman, J. L. & Stone, J. R. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc. Pathol. 43, 107148 (2019).
Bennet, A. M., Alarcon-Riquelme, M., Wiman, B., de Faire, U. & Prokunina-Olsson, L. Decreased risk for myocardial infarction and lower tumor necrosis factor-alpha levels in carriers of variants of the PDCD1 gene. Hum. Immunol. 67, 700–705 (2006).
Yip, H. K. et al. Cytotoxic T lymphocyte antigen 4 gene polymorphism associated with ST-segment elevation acute myocardial infarction. Circ. J. 71, 1213–1218 (2007).
Yin, X. et al. Protein biomarkers of new-onset cardiovascular disease: prospective study from the systems approach to biomarker research in cardiovascular disease initiative. Arterioscler. Thromb. Vasc. Biol. 34, 939–945 (2014).
Forteza, M. J. et al. Programmed death-1 (PD-1): a novel mechanism for understanding the acute immune deregulation in ST-segment elevation myocardial infarction. Int J. Cardiol. 177, 8–10 (2014).
Pasqui, A. L. et al. T cell activation and enhanced apoptosis in non-ST elevation myocardial infarction. Clin. Exp. Med. 3, 37–44 (2003).
Carvalheiro, T. et al. Phenotypic and functional alterations on inflammatory peripheral blood cells after acute myocardial infarction. J. Cardiovasc. Transl. Res. 5, 309–320 (2012).
Hosono, M. et al. Increased expression of T cell activation markers (CD25, CD26, CD40L and CD69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis 168, 73–80 (2003).
Bruestle, K., Hackner, K., Kreye, G. & Heidecker, B. Autoimmunity in acute myocarditis: how immunopathogenesis steers new directions for diagnosis and treatment. Curr. Cardiol. Rep. 22, 28 (2020).
Lv, H. et al. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J. Clin. Invest. 121, 1561–1573 (2011).
Grabie, N., Lichtman, A. H. & Padera, R. T cell checkpoint regulators in the heart. Cardiovasc. Res. 115, 869–877 (2019).
Johnson, D. B. et al. Fulminant myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med 375, 1749–1755 (2016).
Mahmood, S. S. et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 71, 1755–1764 (2018).
Shrestha, K. P. & Carrera, A. E. Hair trace elements and mental retardation among children. Arch. Environ. Health 43, 396–398 (1988).
Moslehi, J. J., Salem, J. E., Sosman, J. A., Lebrun-Vignes, B. & Johnson, D. B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 391, 933 (2018).
Escudier, M. et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 136, 2085–2087 (2017).
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Tarrio, M. L., Grabie, N., Bu, D. X., Sharpe, A. H. & Lichtman, A. H. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J. Immunol. 188, 4876–4884 (2012).
Tivol, E. A. et al. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J. Immunol. 158, 5091–5094 (1997).
Ji, C. et al. Myocarditis in cynomolgus monkeys following treatment with immune checkpoint inhibitors. Clin. Cancer Res. 25, 4735–4748 (2019).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Rodig, N. et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126 (2003).
Koelzer, V. H. et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J. Immunother. Cancer 4, 13 (2016).
Frisancho-Kiss, S. et al. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J. Immunol. 176, 6411–6415 (2006).
Frisancho-Kiss, S. et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 178, 6710–6714 (2007).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Pennock, N. D. et al. T cell responses: naive to memory and everything in between. Adv. Physiol. Educ. 37, 273–283 (2013).
Teng, M. W., Galon, J., Fridman, W. H. & Smyth, M. J. From mice to humans: developments in cancer immunoediting. J. Clin. Invest. 125, 3338–3346 (2015).
Otsuka, A. et al. Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma. Clin. Cancer Res. 21, 1289–1297 (2015).
Vinay, D. S. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35(Suppl), S185–S198 (2015).
Sarvaria, A., Madrigal, J. A. & Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol. 14, 662–674 (2017).
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Baitsch, L. et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Speiser, D. E. et al. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat. Rev. Immunol. 14, 768–774 (2014).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Wolchok, J. D. et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 11, 155–164 (2010).
Eggermont, A. M. et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 16, 522–530 (2015).
Yang, J. C. et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 30, 825–830 (2007).
Ansell, S. M. et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 15, 6446–6453 (2009).
Kwon, E. D. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712 (2014).
Ribas, A. et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31, 616–622 (2013).
Chung, K. Y. et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J. Clin. Oncol. 28, 3485–3490 (2010).
Sangro, B. et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 59, 81–88 (2013).
Calabro, L. et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 14, 1104–1111 (2013).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Page, D. B., Postow, M. A., Callahan, M. K., Allison, J. P. & Wolchok, J. D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 65, 185–202 (2014).
Lipson, E. J. et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 19, 462–468 (2013).
Mahoney, K. M., Freeman, G. J. & McDermott, D. F. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin. Ther. 37, 764–782 (2015).
Berger, R. et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 14, 3044–3051 (2008).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Lynch, T. J. et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 30, 2046–2054 (2012).
Horinouchi, H. et al. Phase I study of ipilimumab in phased combination with paclitaxel and carboplatin in Japanese patients with non-small-cell lung cancer. Invest. N. Drugs 33, 881–889 (2015).
Arriola, E. et al. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J. Thorac. Oncol. 11, 1511–1521 (2016).
Hersh, E. M. et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest N. Drugs 29, 489–498 (2011).
Chi, M. & Dudek, A. Z. Vaccine therapy for metastatic melanoma: systematic review and meta-analysis of clinical trials. Melanoma Res. 21, 165–174 (2011).
Bernatchez, C., Radvanyi, L. G. & Hwu, P. Advances in the treatment of metastatic melanoma: adoptive T-cell therapy. Semin. Oncol. 39, 215–226 (2012).
Boni, A. et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 70, 5213–5219 (2010).
Slovin, S. F. et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann. Oncol. 24, 1813–1821 (2013).
Wilmott, J. S. et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 18, 1386–1394 (2012).
Ribas, A., Hodi, F. S., Callahan, M., Konto, C. & Wolchok, J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 368, 1365–1366 (2013).
Puhr, H. C. & Ilhan-Mutlu, A. New emerging targets in cancer immunotherapy: the role of LAG3. ESMO Open 4, e000482 (2019).
Tagliamento, M., Bironzo, P. & Novello, S. New emerging targets in cancer immunotherapy: the role of VISTA. ESMO Open 4, https://doi.org/10.1136/esmoopen-2020-000683 (2020).
Johnston, R. L., Lutzky, J., Chodhry, A. & Barkin, J. S. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig. Dis. Sci. 54, 2538–2540 (2009).
Abdel-Rahman, O. & Fouad, M. A network meta-analysis of the risk of immune-related renal toxicity in cancer patients treated with immune checkpoint inhibitors. Immunotherapy 8, 665–674 (2016).
Nonomura, Y. et al. ADAMTSL5 is upregulated in melanoma tissues in patients with idiopathic psoriasis vulgaris induced by nivolumab. J. Eur. Acad. Dermatol. Venereol. 31, e100–e101 (2017).
Chae, Y. K. et al. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol. Immunother. 66, 25–32 (2017).
Tarhini, A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Science (Cairo) 2013, 857519 (2013).
Fecher, L. A., Agarwala, S. S., Hodi, F. S. & Weber, J. S. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 18, 733–743 (2013).
Weber, J. S. et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 119, 1675–1682 (2013).
Marthey, L. et al. Cancer Immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J. Crohns Colitis 10, 395–401 (2016).
Kim, K. W. et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest N. Drugs 31, 1071–1077 (2013).
Dillard, T., Yedinak, C. G., Alumkal, J. & Fleseriu, M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 13, 29–38 (2010).
Bertrand, A., Kostine, M., Barnetche, T., Truchetet, M. E. & Schaeverbeke, T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 13, 211 (2015).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375–2391 (2015).
Qin, W. et al. The diverse gunction of PD-1/PD-L pathway beyond vancer. Front. Immunol. 10, 2298 (2019).
Stohl, W., Yu, N., Chalmers, S. A., Putterman, C. & Jacob, C. O. Constitutive reduction in the checkpoint inhibitor, CTLA-4, does not accelerate SLE in NZM 2328 mice. Lupus Sci. Med. 6, e000313 (2019).
Fadel, F., El Karoui, K. & Knebelmann, B. Anti-CTLA4 antibody-induced lupus nephritis. N. Engl. J. Med. 361, 211–212 (2009).
Richter, M. D. et al. Brief Report: Cancer immunotherapy in patients with preexisting rheumatic disease: The Mayo Clinic experience. Arthritis Rheumatol. 70, 356–360 (2018).
Simons, K. H. et al. T cell co-stimulation and co-inhibition in cardiovascular disease: a double-edged sword. Nat. Rev. Cardiol. 16, 325–343 (2019).
Mensah, G. A., Roth, G. A. & Fuster, V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. 74, 2529–2532 (2019).
Esplugues, E. et al. Enhanced antitumor immunity in mice deficient in CD69. J. Exp. Med 197, 1093–1106 (2003).
Cibrian, D. & Sanchez-Madrid, F. CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol. 47, 946–953 (2017).
Song, W., Das, M. & Chen, X. Nanotherapeutics for immuno-oncology: a crossroad for new paradigms. Trends Cancer 6, 288–298 (2020).
Grosser, R., Cherkassky, L., Chintala, N. & Adusumilli, P. S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 36, 471–482 (2019).
Gianchecchi, E. & Fierabracci, A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in Treg development and their involvement in autoimmunity onset and cancer progression. Front. Immunol. 9, 2374 (2018).
Michel, L. et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J. Clin. Invest. 118, 3411–3419 (2008).
Zelle-Rieser, C. et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J. Hematol. Oncol. 9, 116 (2016).
Heinzerling, L. et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Cancer 4, 50 (2016).
Zhang, Z. et al. Two genes encoding immune-regulatory molecules (LAG3 and IL7R) confer susceptibility to multiple sclerosis. Genes Immun. 6, 145–152 (2005).
Pallikkuth, S. et al. Cardiac morbidity in HIV infection is associated with checkpoint inhibitor LAG-3 on CD4 T cells. PLoS One 13, e0206256 (2018).
Liberal, R. et al. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway. Hepatology 56, 677–686 (2012).
Das, M., Zhu, C. & Kuchroo, V. K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 276, 97–111 (2017).
Japp, A. S. et al. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol. Immunother. 64, 1487–1494 (2015).
Agresta, L., Hoebe, K. H. N. & Janssen, E. M. The emerging role of CD244 signaling in immune cells of the tumor microenvironment. Front. Immunol. 9, 2809 (2018).
Peng, J. & Xiang, Y. Value analysis of CD69 combined with EGR1 in the diagnosis of coronary heart disease. Exp. Ther. Med. 17, 2047–2052 (2019).
Wald, O. et al. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J. Immunol. 177, 6983–6990 (2006).
Oja, A. E. et al. Functional heterogeneity of CD4(+) tumor-infiltrating lymphocytes with a resident memory phenotype in NSCLC. Front. Immunol. 9, 2654 (2018).
Yu, X., Zheng, Y., Mao, R., Su, Z. & Zhang, J. BTLA/HVEM signaling: milestones in research and role in chronic hepatitis B virus infection. Front. Immunol. 10, 617 (2019).
Burgueno-Bucio, E., Mier-Aguilar, C. A. & Soldevila, G. The multiple faces of CD5. J. Leukoc. Biol. 105, 891–904 (2019).
Acknowledgements
This study was supported by competitive grants from the Ministerio de Ciencia, Innovación y Universidades, through the Carlos III Institute of Health-Fondo de Investigación Sanitaria (PI19/00545) to P.M.; CIBER Cardiovascular (Fondo de Investigación Sanitaria del Instituto de Salud Carlos III and cofunding by Fondo Europeo de Desarrollo Regional FEDER) to P.M.; and CAM (S2017/BMD-3671-INFLAMUNE-CM) from the Comunidad de Madrid to P.M. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia e Innovación and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015-0505). R.B.-D. is supported by the Formación de Profesorado Universitario (FPU16/02780) program of the Spanish Ministry of Education, Culture and Sports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Martín, P., Blanco-Domínguez, R. & Sánchez-Díaz, R. Novel human immunomodulatory T cell receptors and their double-edged potential in autoimmunity, cardiovascular disease and cancer. Cell Mol Immunol 18, 919–935 (2021). https://doi.org/10.1038/s41423-020-00586-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-020-00586-4
Keywords
This article is cited by
-
ISG20L2: an RNA nuclease regulating T cell activation
Cellular and Molecular Life Sciences (2023)
-
CD69-oxLDL ligand engagement induces Programmed Cell Death 1 (PD-1) expression in human CD4 + T lymphocytes
Cellular and Molecular Life Sciences (2022)