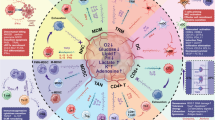
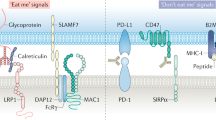
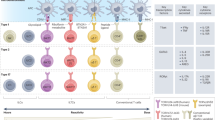
Abstract
The innate immune sensing pathways play critical roles in the defense against pathogen infection, but their roles in cancer immunosurveillance and cancer therapies are less defined. We propose that defective innate immune sensing inside the tumor microenvironment might limit T-cell responses to immunotherapy. A recent mechanistic understanding of conventional therapies revealed that both innate immune sensing and T-cell responses are essential for optimal antitumor efficacy. T-cell-based immunotherapy, particularly immune checkpoint blockade, has achieved great success in reactivating antitumor immune responses to lead to tumor regression, but only in a small fraction of patients. Therefore, incorporating conventional therapy that can increase innate sensing and immunotherapy should lead to promising strategies for cancer patients. Here, we review the innate sensing pathways related to cancer initiation/progression and therapies, summarize the recent key findings in innate immune sensing related to conventional therapies, evaluate current combination strategies, and highlight the potential issues of combinational therapies in terms of antitumor efficacy and toxicities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Janeway, C. A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54(Pt 1), 1–13 (1989).
Takeuchi, O. & Akira, S. Pathogen recognition by innate immunity. Arerugi 56, 558–562 (2007).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Pasare, C. & Medzhitov, R. Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 560, 11–18 (2005).
Rakoff-Nahoum, S. & Medzhitov, R. Toll-like receptors and cancer. Nat. Rev. Cancer 9, 57–63 (2009).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Corrales, L., Matson, V., Flood, B., Spranger, S. & Gajewski, T. F. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 27, 96–108 (2017).
Hamid, O. et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J. Transl. Med. 9, https://doi.org/10.1186/1479-5876-9-204 (2011).
Rusakiewicz, S. et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 73, 3499–3510 (2013).
Mahmoud, S. M. A. et al. Tumor-infiltrating CD8(+) lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 29, 1949–1955 (2011).
Woo, J. W. et al. Tumour-infiltrating CD8+lymphocytes after primary systemic therapy predict clinical outcome in patients with breast cancer. Virchows Arch. 473, S54–S55 (2018).
Azimi, F. et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J. Clin. Oncol. 30, 2678–2683 (2012).
Mlecnik, B. et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 29, 610–618 (2011).
Pages, F. et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29, 1093–1102 (2010).
Bogolyubova, A. V. & Belousov, P. V. Inflammatory immune infiltration in human tumors: role in pathogenesis and prognostic and diagnostic value. Biochemistry 81, 1261–1273 (2016).
Arruebo, M. et al. Assessment of the evolution of cancer treatment therapies. Cancers 3, 3279–3330 (2011).
Baudino, T. A. Targeted cancer therapy: the next generation of cancer treatment. Curr. Drug Discov. Technol. 12, 3–20 (2015).
Padma, V. V. An overview of targeted cancer therapy. Biomedicine 5, 19 (2015).
Bracci, L., Schiavoni, G., Sistigu, A. & Belardelli, F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 21, 15–25 (2014).
Baskar, R., Dai, J., Wenlong, N., Yeo, R. & Yeoh, K. W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 1, 24 (2014).
Liu, Z. et al. Hypofractionated EGFR tyrosine kinase inhibitor limits tumor relapse through triggering innate and adaptive immunity. Sci Immunol 4, https://doi.org/10.1126/sciimmunol.aav6473 (2019).
Park, S. et al. The therapeutic effect of Anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 18, 160–170 (2010).
Deng, L. et al. Damage to nucleic acid sensing: a strategy to enhance radiation therapy. Clin. Cancer Res. 22, 20–25 (2016).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Wargo, J. A., Cooper, Z. A. & Flaherty, K. T. Universes collide: combining immunotherapy with targeted therapy for cancer. Cancer Discov. 4, 1377–1386 (2014).
Ribas, A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 5, 915–919 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Tang, H., Qiao, J. & Fu, Y. X. Immunotherapy and tumor microenvironment. Cancer Lett. 370, 85–90 (2016).
Topalian, S. L. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32, 1020–1030 (2014).
Simone, C. B., Burri, S. H. & Heinzerling, J. H. Novel radiotherapy approaches for lung cancer: combining radiation therapy with targeted and immunotherapies. Transl. Lung Cancer R. 4, 545–552 (2015).
Gotwals, P. et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 17, 286–301 (2017).
Zappasodi, R., Merghoub, T. & Wolchok, J. D. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell 33, 581–598 (2018).
Robert, L., Ribas, A. & Hu-Lieskovan, S. Combining targeted therapy with immunotherapy. Can 1+1 equal more than 2? Semin. Immunol. 28, 73–80 (2016).
Beutler, B. Toll-like receptors: how they work and what they do. Curr. Opin. Hematol. 9, 2–10 (2002).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Kawasaki, T. & Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 5, 461 (2014).
O'Neill, L. A., Fitzgerald, K. A. & Bowie, A. G. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 24, 286–290 (2003).
West, A. P., Koblansky, A. A. & Ghosh, S. Recognition and signaling by toll-like receptors. Annu. Rev. Cell Dev. Biol. 22, 409–437 (2006).
Uematsu, S. & Akira, S. Toll-like receptors and innate immunity. J. Mol. Med. 84, 712–725 (2006).
Cui, J., Chen, Y., Wang, H. Y. & Wang, R. F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccin Immunother. 10, 3270–3285 (2014).
Castano-Rodriguez, N., Kaakoush, N. O. & Mitchell, H. M. Pattern-recognition receptors and gastric cancer. Front. Immunol. 5, 336 (2014).
Huang, B., Zhao, J., Unkeless, J. C., Feng, Z. H. & Xiong, H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene 27, 218–224 (2008).
Luo, J. L., Maeda, S., Hsu, L. C., Yagita, H. & Karin, M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 6, 297–305 (2004).
Harmey, J. H. et al. Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. Int. J. Cancer 101, 415–422 (2002).
Cen, X., Liu, S. & Cheng, K. The role of Toll-like receptor in inflammation and tumor immunity. Front. Pharm. 9, 878 (2018).
Clarke, S. R. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J. Leukoc. Biol. 67, 607–614 (2000).
Chi, H. et al. Anti-tumor Activity of Toll-like receptor 7 agonists. Front. Pharm. 8, 304 (2017).
Vacchelli, E. et al. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology 1, 894–907 (2012).
Hoffman, E. S., Smith, R. E. & Renaud, R. C. Jr. From the analyst's couch: TLR-targeted therapeutics. Nat. Rev. Drug Discov. 4, 879–880 (2005).
Hancz, D. et al. Flagellin increases death receptor-mediated cell death in a RIP1-dependent manner. Immunol. Lett. 193, 42–50 (2018).
Takaki, H., Shime, H., Matsumoto, M. & Seya, T. Tumor cell death by pattern-sensing of exogenous RNA: Tumor cell TLR3 directly induces necroptosis by poly(I:C) in vivo, independent of immune effector-mediated tumor shrinkage. Oncoimmunology 6, e1078968 (2017).
Dambuza, I. M. & Brown, G. D. C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32, 21–27 (2015).
Drickamer, K. & Fadden, A. J. Genomic analysis of C-type lectins. Biochem. Soc. Symp. 69, 59–72 (2002).
Zelensky, A. N. & Gready, J. E. The C-type lectin-like domain superfamily. FEBS J. 272, 6179–6217 (2005).
Hardison, S. E. & Brown, G. D. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 13, 817–822 (2012).
Sancho, D. & Reis e Sousa, C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 30, 491–529 (2012).
Kerrigan, A. M. & Brown, G. D. Syk-coupled C-type lectins in immunity. Trends Immunol. 32, 151–156 (2011).
Del Fresno, C., Iborra, S., Saz-Leal, P., Martinez-Lopez, M. & Sancho, D. Flexible signaling of myeloid C-type lectin receptors in immunity and inflammation. Front. Immunol. 9, 804 (2018).
Drummond, R. A. & Brown, G. D. Signalling C-type lectins in antimicrobial immunity. PLoS Pathog. 9, e1003417 (2013).
Redelinghuys, P. & Brown, G. D. Inhibitory C-type lectin receptors in myeloid cells. Immunol. Lett. 136, 1–12 (2011).
Geijtenbeek, T. B. & Gringhuis, S. I. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9, 465–479 (2009).
Nonaka, M. et al. Glycosylation-dependent interactions of C-type lectin DC-SIGN with colorectal tumor-associated Lewis glycans impair the function and differentiation of monocyte-derived dendritic cells. J. Immunol. 180, 3347–3356 (2008).
Aarnoudse, C. A., Garcia Vallejo, J. J., Saeland, E. & van Kooyk, Y. Recognition of tumor glycans by antigen-presenting cells. Curr. Opin. Immunol. 18, 105–111 (2006).
Leibundgut-Landmann, S., Osorio, F. & Brown, G. D. & Reis e Sousa, C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 112, 4971–4980 (2008).
Napoletano, C. et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 42, 936–945 (2012).
Yan, H., Ohno, N. & Tsuji, N. M. The role of C-type lectin receptors in immune homeostasis. Int. Immunopharmacol. 16, 353–357 (2013).
Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Allavena, P. et al. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin. Dev. Immunol. 2010, 547179 (2010).
Tian, J. et al. Beta-glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur. J. Immunol. 43, 1220–1230 (2013).
Masuda, Y., Inoue, M., Miyata, A., Mizuno, S. & Nanba, H. Maitake beta-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. Int. Immunopharmacol. 9, 620–626 (2009).
Liu, Z., Zhou, H., Wang, W., Fu, Y. X. & Zhu, M. A novel dendritic cell targeting HPV16 E7 synthetic vaccine in combination with PD-L1 blockade elicits therapeutic antitumor immunity in mice. Oncoimmunology 5, e1147641 (2016).
Liu, Z. et al. A novel method for synthetic vaccine construction based on protein assembly. Sci. Rep. 4, 7266 (2014).
Park, H. Y. et al. Enhancing vaccine antibody responses by targeting Clec9A on dendritic cells. NPJ Vaccines 2, 31 (2017).
Tacken, P. J., Torensma, R. & Figdor, C. G. Targeting antigens to dendritic cells in vivo. Immunobiology 211, 599–608 (2006).
Meylan, E., Tschopp, J. & Karin, M. Intracellular pattern recognition receptors in the host response. Nature 442, 39–44 (2006).
Elinav, E., Strowig, T., Henao-Mejia, J. & Flavell, R. A. Regulation of the antimicrobial response by NLR proteins. Immunity 34, 665–679 (2011).
Saxena, M. & Yeretssian, G. NOD-like receptors: master regulators of inflammation and cancer. Front. Immunol. 5, 327 (2014).
Chen, G., Shaw, M. H., Kim, Y. G. & Nunez, G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 4, 365–398 (2009).
Franchi, L., Munoz-Planillo, R. & Nunez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13, 325–332 (2012).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Agostini, L. et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Couturier-Maillard, A. et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Investig. 123, 700–711 (2013).
Chen, G. Y., Shaw, M. H., Redondo, G. & Nunez, G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 68, 10060–10067 (2008).
Millrud, C. R. et al. Nod-like receptors in head and neck squamous cell carcinoma. Acta Otolaryngol. 133, 1333–1344 (2013).
Cook, G. P., Savic, S., Wittmann, M. & McDermott, M. F. The NLRP3 inflammasome, a target for therapy in diverse disease states. Eur. J. Immunol. 40, 631–634 (2010).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Chow, M. T. et al. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 72, 5721–5732 (2012).
Wei, Q. et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab. Investig. 94, 52–62 (2014).
Zaki, M. H. et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 20, 649–660 (2011).
Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl Acad. Sci. USA 108, 9601–9606 (2011).
Hu, B. et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl Acad. Sci. USA 107, 21635–21640 (2010).
Paludan, S. R. & Bowie, A. G. Immune sensing of DNA. Immunity 38, 870–880 (2013).
Yoneyama, M. et al. Viral RNA dedetection by RIG-I-like receptors. Curr. Opin. Immunol. 32, 48–53 (2015).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Wu, J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013).
Ferguson, B. J., Mansur, D. S., Peters, N. E., Ren, H. & Smith, G. L. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. eLife 1, e00047 (2012).
Schlee, M. Master sensors of pathogenic RNA - RIG-I like receptors. Immunobiology 218, 1322–1335 (2013).
Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Duewell, P. et al. RIG-I-like helicases induce immunogenic cell death of pancreatic cancer cells and sensitize tumors toward killing by CD8(+) T cells. Cell Death Differ. 21, 1825–1837 (2014).
Bu, Y., Liu, F., Jia, Q. A. & Yu, S. N. Decreased expression of TMEM173 predicts poor prognosis in patients with hepatocellular carcinoma. PLoS ONE 11, e0165681 (2016).
Lemos, H. et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 76, 2076–2081 (2016).
Hu, J. et al. Dose dependent activation of retinoic acid-inducible gene-I promotes both proliferation and apoptosis signals in human head and neck squamous cell carcinoma. PLoS ONE 8, e58273 (2013).
Pestka, S., Krause, C. D. & Walter, M. R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32 (2004).
Fuertes, M. B., Woo, S. R., Burnett, B., Fu, Y. X. & Gajewski, T. F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 34, 67–73 (2013).
Rathinam, V. A. & Fitzgerald, K. A. Cytosolic surveillance and antiviral immunity. Curr. Opin. Virol. 1, 455–462 (2011).
Platanias, L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005).
Hervas-Stubbs, S. et al. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 17, 2619–2627 (2011).
Lorenzi, S. et al. Type I IFNs control antigen retention and survival of CD8alpha(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J. Immunol. 186, 5142–5150 (2011).
Curtsinger, J. M. et al. IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 174, 4465–4469 (2005).
Dunn, G. P., Koebel, C. M. & Schreiber, R. D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Dunn, G. P. et al. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 6, 722–729 (2005).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Medrano, R. F. V., Hunger, A., Mendonca, S. A., Barbuto, J. A. M. & Strauss, B. E. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget 8, 71249–71284 (2017).
Moschos, S. J. et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J. Clin. Oncol. 24, 3164–3171 (2006).
Liang, Y. et al. Targeting IFNalpha to tumor by anti-PD-L1 creates feedforward antitumor responses to overcome checkpoint blockade resistance. Nat. Commun. 9, 4586 (2018).
Yang, X. et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell 25, 37–48 (2014).
Michelle Xu, M., Pu, Y., Weichselbaum, R. R. & Fu, Y. X. Integrating conventional and antibody-based targeted anticancer treatment into immunotherapy. Oncogene 36, 585–592 (2017).
Xu, M. M., Pu, Y., Zhang, Y. & Fu, Y. X. The Role of Adaptive Immunity in the Efficacy of Targeted Cancer Therapies. Trends Immunol. 37, 141–153 (2016).
Baskar, R., Lee, K. A., Yeo, R. & Yeoh, K.-W. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 9, 193 (2012).
Hoskin, P. J. & Bhattacharya, I. S. Protons and more: state of the art in radiotherapy. Clin. Med. 14, s61–s65 (2014).
Srinivas, U. S., Tan, B., Vellayappan, B. A. & Jeyasekharan, A. D. ROS and the DNA damage response in cancer. Redox biology.25, 101084 (2018).
Lee, Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009).
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860 (2012).
Wu, Y., Wu, X., Wu, L., Wang, X. & Liu, Z. The anticancer functions of RIG-I–like receptors, RIG-I and MDA5, and their applications in cancer therapy. Transl. Res. 190, 51–60 (2017).
Ranoa, D. R. E. et al. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget 7, 26496 (2016).
Burnette, B. C. et al. The efficacy of radiotherapy relies upon induction of type I interferon–dependent innate and adaptive immunity. Cancer Res. 71, 2488–2496 (2011).
Harding, S. M. et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466 (2017).
Mackenzie, K. J. et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461 (2017).
de Oliveira Mann, C. C. & Kranzusch, P. J. cGAS conducts micronuclei DNA surveillance. Trends Cell Biol. 27, 697–698 (2017).
Vanpouille-Box, C. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618 (2017).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Heid, M. E. et al. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol. 191, 5230–5238 (2013).
Hu, B. et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 354, 765–768 (2016).
Liu, Y. G. et al. NLRP3 inflammasome activation mediates radiation-induced pyroptosis in bone marrow-derived macrophages. Cell Death Dis. 8, e2579 (2017).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Veeranki, S., Duan, X., Panchanathan, R., Liu, H. & Choubey, D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PLoS ONE 6, e27040 (2011).
Henry, T., Brotcke, A., Weiss, D. S., Thompson, L. J. & Monack, D. M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007).
Gaidt, M. M. et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell 171, 1110–1124. e1118 (2017).
Wang, Y. et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity 46, 393–404 (2017).
Banerjee, I. et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity 49, 413–426. e415 (2018).
Servomaa, K. & Rytömaa, T. UV light and ionizing radiations cause programmed death of rat chloroleukaemia cells by inducing retropositions of a mobile DNA element (L1Rn). Int. J. Radiat. Biol. 57, 331–343 (1990).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Lang, X. et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-19-0338 (2019).
Wang, W. et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019).
Garg, A. D. et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front. Immunol. 6, 588 (2015).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu Rev. Immunol. 31, 51–72 (2013).
Zhou, J. et al. Immunogenic cell death in cancer therapy: present and emerging inducers. J. Cell Mol. Med. 23, 4854–4865 (2019).
Zitvogel, L., Galluzzi, L., Smyth, M. J. & Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39, 74–88 (2013).
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Bruchard, M. et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 19, 57–64 (2013).
Pescovitz, M. D. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am. J. Transpl. 6, 859–866 (2006).
Baselga, J. & Albanell, J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann. Oncol. 12(Suppl 1), S35–S41, https://doi.org/10.1093/annonc/12.suppl_1.s35 (2001).
Valabrega, G., Montemurro, F. & Aglietta, M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 18, 977–984 (2007).
Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 6, 443–446 (2000).
Maloney, D. G. Anti-CD20 antibody therapy for B-cell lymphomas. N. Engl. J. Med. 366, 2008–2016 (2012).
Ren, Z. et al. CTLA-4 limits anti-CD20-mediated tumor regression. Clin. Cancer Res. 23, 193–203 (2017).
Dominguez, C., Tsang, K. Y. & Palena, C. Short-term EGFR blockade enhances immune-mediated cytotoxicity of EGFR mutant lung cancer cells: rationale for combination therapies. Cell Death Dis. 7, e2380 (2016).
Arnould, L. et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer 94, 259–267 (2006).
Chao, M. P. et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 (2010).
Stagg, J. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA 108, 7142–7147 (2011).
Correale, P. et al. Cetuximab +/− chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int. J. Cancer 130, 1577–1589 (2012).
Pozzi, C. et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat. Med. 22, 624–631 (2016).
Liu, J. et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE 10, e0137345 (2015).
Majeti, R. et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, 286–299 (2009).
Liu, X. et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 21, 1209–1215 (2015).
Arslan, M. A., Kutuk, O. & Basaga, H. Protein kinases as drug targets in cancer. Curr. Cancer Drug Tar. 6, 623–634 (2006).
Yamaoka, T., Ohba, M. & Ohmori, T. Molecular-targeted therapies for epidermal growth factor receptor and its resistance mechanisms. Int. J. Mol. Sci .18, https://doi.org/10.3390/ijms18112420 (2017).
Ciardiello, F. & Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 358, 1160–1174 (2008).
Sequist, L. V. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31, 3327–3334 (2013).
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Xu, M., Xie, Y., Ni, S. & Liu, H. The latest therapeutic strategies after resistance to first generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in patients with non-small cell lung cancer (NSCLC). Ann. Transl. Med. 3, 96 (2015).
Stewart, E. L., Tan, S. Z., Liu, G. & Tsao, M. S. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl. Lung Cancer Res. 4, 67–81 (2015).
Friess, T., Scheuer, W. & Hasmann, M. Erlotinib antitumor activity in non-small cell lung cancer models is independent of HER1 and HER2 overexpression. Anticancer Res. 26, 3505–3512 (2006).
Ioannou, N. et al. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. Br. J. Cancer 105, 1554–1562 (2011).
Cross, D. A. et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 4, 1046–1061 (2014).
Kumai, T. et al. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br. J. Cancer 109, 2155–2166 (2013).
Yakes, F. M. et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 10, 2298–2308 (2011).
Verzoni, E. et al. Potent natural killer (NK) and myeloid blood cell remodeling by cabozantinib (Cabo) in pre-treated metastatic renal cell carcinoma (mRCC) patients. Ann. Oncol. 29, https://doi.org/10.1093/annonc/mdy283.091 (2018).
Lu, X. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017).
Patnaik, A. et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, other solid tumors. Cancer Discov. 6, 740–753 (2016).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Lord, C. J. & Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 355, 1152–1158 (2017).
Ding, L. et al. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. 25, 2972–2980 e2975 (2018).
Pantelidou, C. et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative. Breast Cancer Cancer Discov. 9, 722–737 (2019).
Choi, Y. J. et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell 22, 438–451 (2012).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471 (2017).
Li, R., Bianchet, M. A., Talalay, P. & Amzel, L. M. The three-dimensional structure of NAD(P)H:quinone reductase, a flavoprotein involved in cancer chemoprotection and chemotherapy: mechanism of the two-electron reduction. Proc. Natl Acad. Sci. USA 92, 8846–8850 (1995).
Oh, E. T. et al. NQO1 inhibits proteasome-mediated degradation of HIF-1alpha. Nat. Commun. 7, 13593 (2016).
Doskey, C. M. et al. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol. 10, 274–284 (2016).
Huang, X. M. et al. Leveraging an NQO1 bioactivatable drug for tumor-selective use of poly(ADP-ribose) polymerase inhibitors. Cancer Cell 30, 940–952 (2016).
Li, X. G. et al. NQO1 targeting prodrug triggers innate sensing to overcome checkpoint blockade resistance. Nat. Commun. 10, 3251 (2019).
Sambi, M., Bagheri, L. & Szewczuk, M. R. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J. Oncol. 2019, 4508794 (2019).
Couzin-Frankel, J. Breakthrough of the year 2013. Cancer Immunother. Sci. 342, 1432–1433 (2013).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Feins, S., Kong, W., Williams, E. F., Milone, M. C. & Fraietta, J. A. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol. 94, S3–S9 (2019).
Guo, C. et al. Therapeutic cancer vaccines: past, present, and future. Adv. Cancer Res. 119, 421–475 (2013).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Larkin, J., Hodi, F. S. & Wolchok, J. D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 1270–1271 (2015).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Huang, A. C. et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017).
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Qiao, J., Liu, Z. & Fu, Y. X. Adapting conventional cancer treatment for immunotherapy. J. Mol. Med. 94, 489–495 (2016).
Shinohara, Y. & Tsukimoto, M. Adenine nucleotides attenuate murine T cell activation induced by concanavalin A or T cell receptor stimulation. Front. Pharm. 8, 986 (2017).
la Sala, A. et al. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 166, 1611–1617 (2001).
Aymeric, L. et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 70, 855–858 (2010).
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 124, 687–695 (2014).
Muroyama, Y. et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol. Res. 5, 992–1004 (2017).
Liang, H. et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 8, 1736 (2017).
Lee, Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009).
Gupta, A. et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 189, 558–566 (2012).
Weiss, T. et al. NKG2D-dependent antitumor effects of chemotherapy and radiotherapy against glioblastoma. Clin. Cancer Res. 24, 882–895 (2018).
Dhar, P. & Wu, J. D. NKG2D and its ligands in cancer. Curr. Opin. Immunol. 51, 55–61 (2018).
Deng, L. et al. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 124, 687–695 (2014).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Demaria, S. & Formenti, S. C. Role of T lymphocytes in tumor response to radiotherapy. Front. Oncol. 2, 95 (2012).
Filatenkov, A. et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 21, 3727–3739 (2015).
Schaue, D., Kachikwu, E. L. & McBride, W. H. Cytokines in radiobiological responses: a review. Radiat. Res. 178, 505–523 (2012).
Marvel, D. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Investig. 125, 3356–3364 (2015).
Movahedi, K. et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell–suppressive activity. Blood 111, 4233–4244 (2008).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009).
Lindau, D., Gielen, P., Kroesen, M., Wesseling, P. & Adema, G. J. The immunosuppressive tumour network: myeloid‐derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138, 105–115 (2013).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti–CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Park, S. S. et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol. Res. 3, 610–619 (2015).
Rodríguez-Ruiz, M. E., Vanpouille-Box, C., Melero, I., Formenti, S. C. & Demaria, S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 39, 644–655 (2018).
Luo, M. et al. Synergistic STING activation by PC7A nanovaccine and ionizing radiation improves cancer immunotherapy. J. Control Release 300, 154–160 (2019).
Kwon, E. D. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712 (2014).
Gong, J., Le, T. Q., Massarelli, E., Hendifar, A. E. & Tuli, R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J. Immunother. Cancer 6, 46 (2018).
Sim, A. J. et al. Radiation therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-cell lymphoma. Int. J. Radiat. Oncol. Biol. Phys. https://doi.org/10.1016/j.ijrobp.2019.05.065 (2019).
Minn, I., Rowe, S. P., Pomper, M. G. & Enhancing, C. A. R. T-cell therapy through cellular imaging and radiotherapy. Lancet Oncol. 20, e443–e451 (2019).
Newcomb, E. W. et al. Radiotherapy enhances antitumor effect of anti-CD137 therapy in a mouse Glioma model. Radiat. Res. 173, 426–432 (2010).
Shi, W. & Siemann, D. W. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res. 26, 3445–3453 (2006).
Demaria, S., Pilones, K. A., Vanpouille-Box, C., Golden, E. B. & Formenti, S. C. The optimal partnership of radiation and immunotherapy: from preclinical studies to clinical translation. Radiat. Res. 182, 170–181 (2014).
Foote, J. B. et al. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol. Res. 5, 468–479 (2017).
Sivick, K. E. et al. Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 25, 3074–3085. e3075 (2018).
Singh, V. K. et al. CBLB613: a TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat. Res. 177, 628–642 (2011).
Dovedi, S. J. et al. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood 121, 251–259 (2013).
Roses, R. E., Xu, M., Koski, G. K. & Czerniecki, B. J. Radiation therapy and Toll-like receptor signaling: implications for the treatment of cancer. Oncogene 27, 200 (2008).
Bryniarski, K., Szczepanik, M., Ptak, M., Zemelka, M. & Ptak, W. Influence of cyclophosphamide and its metabolic products on the activity of peritoneal macrophages in mice. Pharm. Rep. 61, 550–557 (2009).
Liu, P., Jaffar, J., Hellstrom, I. & Hellstrom, K. E. Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J. Immunother. 33, 53–59 (2010).
Ghiringhelli, F. et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 56, 641–648 (2007).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714 (2015).
Zielinski, C., Knapp, S., Mascaux, C. & Hirsch, F. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann. Oncol. 24, 1170–1179 (2013).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Liu, Z. et al. Coordinating antigen cytosolic delivery and danger signaling to program potent cross-priming by micelle-based nanovaccine. Cell Discov. 3, 17007 (2017).
Brea, E. J. et al. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol. Res. 4, 936–947 (2016).
Boni, A. et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 70, 5213–5219 (2010).
Ebert, P. J. R. et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44, 609–621 (2016).
Callahan, M. K. et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol. Res. 2, 70–79 (2014).
Hu-Lieskovan, S. et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci. Transl. Med. 7, 279ra241 (2015).
Lowe, D. B. et al. Dasatinib promotes the expansion of a therapeutically superior T-cell repertoire in response to dendritic cell vaccination against melanoma. Oncoimmunology 3, e27589 (2014).
Terme, M. et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 73, 539–549 (2013).
Desar, I. M. et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. Int. J. Cancer 129, 507–512 (2011).
Huang, H. et al. VEGF suppresses T-lymphocyte infiltration in the tumor microenvironment through inhibition of NF-kappaB-induced endothelial activation. FASEB J. 29, 227–238 (2015).
Ahn, M. J. et al. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin. Drug Saf. 16, 465–469 (2017).
Sun, Z. et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat. Commun. 10, 3874 (2019).
Acknowledgements
We thank Casey Moore for helpful editing and discussions. Y.-X.F. holds the Mary Nell and Ralph B. Rogers Professorship in Immunology. This work was supported in part by Texas CPRIT grants RP180725 and RR150072 (CPRIT scholar in Cancer Research) to Y.-X.F.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Liu, Z., Han, C. & Fu, YX. Targeting innate sensing in the tumor microenvironment to improve immunotherapy. Cell Mol Immunol 17, 13–26 (2020). https://doi.org/10.1038/s41423-019-0341-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0341-y
Keywords
This article is cited by
-
The potential applications of microparticles in the diagnosis, treatment, and prognosis of lung cancer
Journal of Translational Medicine (2022)
-
Blockade of checkpoint receptor PVRIG unleashes anti-tumor immunity of NK cells in murine and human solid tumors
Journal of Hematology & Oncology (2021)
-
Small molecular drugs reshape tumor microenvironment to synergize with immunotherapy
Oncogene (2021)
-
Distinct roles but cooperative effect of TLR3/9 agonists and PD-1 blockade in converting the immunotolerant microenvironment of irreversible electroporation-ablated tumors
Cellular & Molecular Immunology (2021)
-
The innate immune effector ISG12a promotes cancer immunity by suppressing the canonical Wnt/β-catenin signaling pathway
Cellular & Molecular Immunology (2020)