Abstract
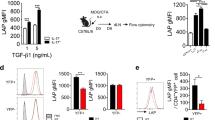
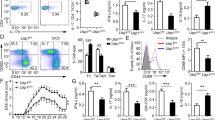
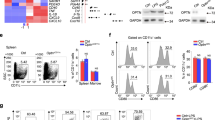
Interleukin-17A (IL-17A)-producing helper T (Th17) cells are a subset of CD4+ T cells that play important pathological roles in autoimmune diseases. Although the intrinsic pathways of Th17 cell differentiation have been well described, how instructive signals derived from the innate immune system trigger the Th17 response and inflammation remains poorly understood. Here, we report that mice deficient in REGγ, a proteasome activator belonging to the 11S family, exhibit significantly deteriorated autoimmune neuroinflammation in an experimental autoimmune encephalomyelitis (EAE) model with augmented Th17 cell polarization in vivo. The results of the adoptive transfer of CD4+ T cells or dendritic cells (DCs) suggest that this phenotype is driven by DCs rather than T cells. Furthermore, REGγ deficiency promotes the expression of integrin αvβ8 on DCs, which activates the maturation of TGF-β1 to enhance Th17 cell development. Mechanistically, this process is mediated by the REGγ-proteasome-dependent degradation of IRF8, a transcription factor for αvβ8. Collectively, our findings delineate a previously unknown mechanism by which REGγ-mediated protein degradation in DCs controls the differentiation of Th17 cells and the onset of an experimental autoimmune disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Weaver, C. T., Hatton, R. D., Mangan, P. R. & Harrington, L. E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852 (2007).
Van Kaer, L., Postoak, J. L., Wang, C., Yang, G. & Wu, L. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol. Immunol. 16, 531–539 (2019).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Chung, Y. et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30, 576–587 (2009).
Ivanov, I. I. et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448, 484–487 (2007).
Mangan, P. R. et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441, 231–234 (2006).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Yang, X. O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28, 29–39 (2008).
Yang, X. O. et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363 (2007).
Chen, W. et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Littman, D. R. & Rudensky, A. Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Korn, T. et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 105, 18460–18465 (2008).
Zhou, L. et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240 (2008).
Ganguly, D., Haak, S., Sisirak, V. & Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 13, 566–577 (2013).
Heink, S. et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 18, 74–85 (2017).
Huang, G. et al. Signaling via the kinase p38alpha programs dendritic cells to drive TH17 differentiation and autoimmune inflammation. Nat. Immunol. 13, 152–161 (2012).
Chen, X., Barton, L. F., Chi, Y., Clurman, B. E. & Roberts, J. M. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol. Cell 26, 843–852 (2007).
Li, X. et al. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol. Cell 26, 831–842 (2007).
Li, X. et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell 124, 381–392 (2006).
Moriishi, K. et al. Critical role of PA28gamma in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 104, 1661–1666 (2007).
Sun, J. et al. The 11S proteasome subunit PSME3 is a positive feedforward regulator of NF-kappaB and important for host defense against bacterial pathogens. Cell Rep. 14, 737–749 (2016).
Xu, J. et al. The REGgamma-proteasome forms a regulatory circuit with IkappaBvarepsilon and NFkappaB in experimental colitis. Nat. Commun. 7, 10761 (2016).
Barton, L. F. et al. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J. Immunol. 172, 3948–3954 (2004).
Uchimura, Y., Barton, L. F., Rada, C. & Neuberger, M. S. REG-gamma associates with and modulates the abundance of nuclear activation-induced deaminase. J. Exp. Med. 208, 2385–2391 (2011).
Gao, G. et al. Proteasome activator REGgamma enhances coxsackieviral infection by facilitating p53 degradation. J. Virol. 84, 11056–11066 (2010).
Bettelli, E., Oukka, M. & Kuchroo, V. K. T(H)-17cells in the circle of immunity and autoimmunity. Nat. Immunol. 8, 345–350 (2007).
Codarri, L. et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
Croxford, A. L. et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43, 502–514 (2015).
Yogev, N. et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity 37, 264–275 (2012).
Jung, S., Unutmaz, D., Wong, P., Sano, G., De los Santos, K. & Sparwasser, T. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Travis, M. A. & Sheppard, D. TGF-beta activation and function in immunity. Annu. Rev. Immunol. 32, 51–82 (2014).
Acharya, M. et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J. Clin. Invest. 120, 4445–4452 (2010).
Melton, A. C. et al. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J. Clin. Invest. 120, 4436–4444 (2010).
Yoshida, Y. et al. The transcription factor IRF8 activates integrin-mediated TGF-beta signaling and promotes neuroinflammation. Immunity 40, 187–198 (2014).
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014).
Kathania, M. et al. Itch inhibits IL-17-mediated colon inflammation and tumorigenesis by ROR-gammat ubiquitination. Nat. Immunol. 17, 997–1004 (2016).
Matsui-Hasumi, A. et al. E3 ubiquitin ligases SIAH1/2 regulate hypoxia-inducible factor-1 (HIF-1)-mediated Th17 cell differentiation. Int. Immunol. 29, 133–143 (2017).
Tanaka, T. et al. PDLIM2 inhibits T helper 17 cell development and granulomatous inflammation through degradation of STAT3. Sci. Signal. 4, ra85 (2011).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Ghoreschi, K. et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467, 967–971 (2010).
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
Stockinger, B. & Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 17, 535–544 (2017).
Yang, Y. et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 206, 1549–1564 (2009).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
El-Behi, M. et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
De Jager, P. L. et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat. Genet. 41, 776–782 (2009).
Xiong, H. et al. Ubiquitin-dependent degradation of interferon regulatory factor-8 mediated by Cbl down-regulates interleukin-12 expression. J. Biol. Chem. 280, 23531–23539 (2005).
Acknowledgements
We thank Dr. Nan Shen for providing CD11c-DTR mice, Dr. Hongyan Wang for providing OT-II mice, and Dr. Stephen L. Nishimura for providing anti-αvβ8 neutralizing antibody. We also thank the ECNU Multifunctional Platform for Innovation (011) for maintaining and raising the mice. This work was supported by the National Program on Key Basic Research Project (2015CB901402), the National Natural Science Foundation of China (31670882, 31730017, 81672883), the Science and Technology Commission of Shanghai Municipality (16ZR1410000, 16QA1401500), and the Foundation of Guangdong Second Provincial General Hospital (2017-001).
Author information
Authors and Affiliations
Contributions
L.Z., X.L. and B.Z. designed the research. L.Z., L.Y. and Q.Z. performed most molecular, cell biology, immunological, and animal experiments. W.X., X.W., J.X., Q.L., Q.L., Y.X., H.Z., L.J., L.W., Weicang Wang, Weichao Wang, and T.S. helped with the experiments. L.F., B.Z., S.L. and L.L. provided scientific advice and valuable expertise. XT.L., B.Z. and L.Z. wrote the manuscript with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhou, L., Yao, L., Zhang, Q. et al. REGγ controls Th17 cell differentiation and autoimmune inflammation by regulating dendritic cells. Cell Mol Immunol 17, 1136–1147 (2020). https://doi.org/10.1038/s41423-019-0287-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0287-0
Key words
This article is cited by
-
The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient "Yin-Yang" theory and modern immunology
Cell Communication and Signaling (2024)
-
Early Growth Response Gene-1 Deficiency Interrupts TGFβ1 Signaling Activation and Aggravates Neurodegeneration in Experimental Autoimmune Encephalomyelitis Mice
Neuroscience Bulletin (2024)
-
The proteasome activator REGγ accelerates cardiac hypertrophy by declining PP2Acα–SOD2 pathway
Cell Death & Differentiation (2020)