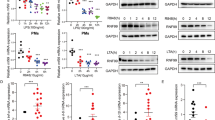
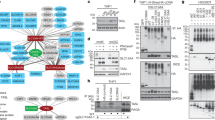
Abstract
Upon recognition of dsRNA, toll-like receptor 3 (TLR3) recruits the adaptor protein TRIF to activate IRF3 and NF-κB signaling, initiating innate immune responses. The ubiquitination of TLR3 downstream signaling molecules and their roles in the innate response have been discovered; however, whether TLR3 itself is ubiquitinated and then functionally involved remains to be elucidated. By immunoprecipitating TLR3-binding proteins in macrophages, we identified ring finger protein 170 (RNF170) as a TLR3-binding E3 ligase. RNF170 mediated the K48-linked polyubiquitination of K766 in the TIR domain of TLR3 and promoted the degradation of TLR3 through the proteasome pathway. The genetic ablation of RNF170 selectively augmented TLR3-triggered innate immune responses both in vitro and in vivo. Our results reveal a novel role for RNF170 in selectively inhibiting TLR3-triggered innate immune responses by promoting TLR3 degradation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Liu, L. et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320, 379–381 (2008).
McGettrick, A. F. & O’Neill, L. A. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr. Opin. Immunol. 22, 20–27 (2010).
Garcia-Cattaneo, A. et al. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc. Natl Acad. Sci. USA 109, 9053–9058 (2012).
Leonard, J. N. et al. The TLR3 signaling complex forms by cooperative receptor dimerization. Proc. Natl Acad. Sci. USA 105, 258–263 (2008).
Roers, A., Hiller, B. & Hornung, V. Recognition of endogenous nucleic acids by the innate immune system. Immunity 44, 739–754 (2016).
Daffis, S., Samuel, M. A., Suthar, M. S., Gale, M. & Diamond, M. S. Toll-Like receptor 3 has a protective role against West nile virus infection. J. Virol. 82, 10349–10358 (2008).
Guillot, L. et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280, 5571–5580 (2005).
Lafaille, F. G. et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491, 769–773 (2012).
Zhang, S. Y. et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317, 1522–1527 (2007).
Harii, N. et al. Thyrocytes express a functional toll-like receptor 3: overexpression can be induced by viral infection and reversed by phenylmethimazole and is associated with Hashimoto’s autoimmune thyroiditis. Mol. Endocrinol. 19, 1231–1250 (2005).
Roelofs, M. F. et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 52, 2313–2322 (2005).
Kondo, T., Kawai, T. & Akira, S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 33, 449–458 (2012).
Liu, J., Qian, C. & Cao, X. Post-translational modification control of innate immunity. Immunity 45, 15–30 (2016).
Cao, X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 16, 35–50 (2016).
Jiang, X. & Chen, Z. J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 12, 35–48 (2011).
Ko, R., Park, J. H., Ha, H., Choi, Y. & Lee, S. Y. Glycogen synthase kinase 3beta ubiquitination by TRAF6 regulates TLR3-mediated pro-inflammatory cytokine production. Nat. Commun. 6, 6765 (2015).
Siednienko, J. et al. Pellino3 targets the IRF7 pathway and facilitates autoregulation of TLR3- and viral-induced expression of type I interferons. Nat. Immunol. 13, 1055–1062 (2012).
Yang, Y. et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc. Natl Acad. Sci. USA 110, 5115–5120 (2013).
Lu, J. P., Wang, Y., Sliter, D. A., Pearce, M. M. & Wojcikiewicz, R. J. RNF170 protein, an endoplasmic reticulum membrane ubiquitin ligase, mediates inositol 1,4,5-trisphosphate receptor ubiquitination and degradation. J. Biol. Chem. 286, 24426–24433 (2011).
Wang, W. et al. RNF122 suppresses antiviral type I interferon production by targeting RIG-I CARDs to mediate RIG-I degradation. Proc. Natl Acad. Sci. USA 113, 9581–9586 (2016).
Wright, F. A. et al. A point mutation in the ubiquitin ligase RNF170 that causes autosomal dominant sensory ataxia destabilizes the protein and impairs inositol 1,4,5-trisphosphate receptor-mediated Ca2+signaling. J. Biol. Chem. 290, 13948–13957 (2015).
Chuang, T. H. & Ulevitch, R. J. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat. Immunol. 5, 495–502 (2004).
Kumazoe, M. et al. Green Tea polyphenol epigallocatechin-3-gallate suppresses toll-like receptor 4 expression via up-regulation of E3 ubiquitin-protein ligase RNF216. J. Biol. Chem. 292, 4077–4088 (2017).
McKelvey, A. C. et al. RING finger E3 ligase PPP1R11 regulates TLR2 signaling and innate immunity. Elife 5, e18496 (2016).
Wang, Y. et al. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 110, 962–971 (2007).
Blasius, A. L. & Beutler, B. Intracellular toll-like receptors. Immunity 32, 305–315 (2010).
Schroder, M. & Bowie, A. G. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 26, 462–468 (2005).
Schulz, O. et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 (2005).
Cario, E. & Podolsky, D. K. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68, 7010–7017 (2000).
Tilton, J. C. et al. Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J. Virol. 80, 11486–11497 (2006).
Hertel, L. & Mocarski, E. S. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of Pseudomitosis independent of US28 function. J. Virol. 78, 11988–12011 (2004).
Netea, M. G., Wijmenga, C. & O’Neill, L. A. Genetic variation in Toll-like receptors and disease susceptibility. Nat. Immunol. 13, 535–542 (2012).
Tabeta, K. et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7, 156–164 (2006).
Brinkmann, M. M. et al. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 177, 265–275 (2007).
Kim, Y. M., Brinkmann, M. M., Paquet, M. E. & Ploegh, H. L. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452, 234–238 (2008).
Kim, Y. et al. Age-dependent gait abnormalities in mice lacking the Rnf170 gene linked to human autosomal-dominant sensory ataxia. Hum. Mol. Genet. 24, 7196–7206 (2015).
Valdmanis, P. N. et al. A mutation in the RNF170 gene causes autosomal dominant sensory ataxia. Brain 134, 602–607 (2011).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant 81788101 to X.C.; grant 81871236 to M.J.), the National 135 Mega Program of China (grant 2017ZX10202203-002 to M.J.; grant 2017ZX10203206-001 to X.C.), the CAMS Innovation Fund for Medical Sciences (grant 2016-12M-1-003 to X.C.) and the Medical Epigenetics Research Center, Chinese Academy of Medical Sciences (2018PT31015).
Author information
Authors and Affiliations
Contributions
M.J. and X.C. designed the research; X.S., M.J., S.L., W.W. and Z.M. performed the experiments; and X.S., M.J. and X.C. analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Song, X., Liu, S., Wang, W. et al. E3 ubiquitin ligase RNF170 inhibits innate immune responses by targeting and degrading TLR3 in murine cells. Cell Mol Immunol 17, 865–874 (2020). https://doi.org/10.1038/s41423-019-0236-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0236-y
Keywords
This article is cited by
-
The RING finger protein family in health and disease
Signal Transduction and Targeted Therapy (2022)