Abstract
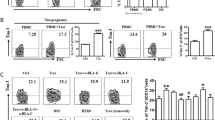
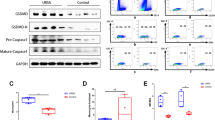
Mesenchymal stem cells (MSCs), which are pluripotent cells with immunomodulatory properties, have been considered good candidates for the therapy of several immune disorders, such as inflammatory bowel diseases, concanavalin A-induced liver injury, and graft-versus-host disease. The embryo is a natural allograft to the maternal immune system. A successful pregnancy depends on the timely extinction of the inflammatory response induced by embryo implantation, followed by the switch to a tolerant immune microenvironment in both the uterus and the system. Excessive infiltration of immune cells and serious inflammatory responses are triggers for embryo rejection, which results in miscarriage. Here, we demonstrated that adoptive transfer of MSCs could prevent fetal loss in a lipopolysaccharide (LPS)-induced abortion model and immune response-mediated spontaneous abortion model. The immunosuppressive MSCs alleviated excessive inflammation by inhibiting CD4 + T cell proliferation and promoting the decidual macrophage switch to M2 in a tumor necrosis factor-stimulated gene-6 (TSG-6)-dependent manner. Cell-to-cell contact with proinflammatory macrophages increased the TSG-6 production by the MSCs, thereby enhancing the suppressive regulation of T cells and macrophages. Moreover, proinflammatory macrophages in contact with the MSCs upregulated the expression of CD200 on the stem cells and facilitated the reprogramming of macrophages towards an anti-inflammatory skew through the interaction of CD200 with CD200R on proinflammatory macrophages. Therefore, the results demonstrate that a TSG-6-mediated paracrine effect, reinforced by cell-to-cell contact between MSCs and proinflammatory macrophages, is involved in the mechanism of MSC-mediated abortion relief through the induction of immune tolerance. Our study also indicates the potential application of MSCs in clinical recurrent miscarriages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Peng, Y. et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia 29, 636–646 (2015).
Dave, M. et al. Stem cells for murine interstitial cells of cajal suppress cellular immunity and colitis via prostaglandin E2 secretion. Gastroenterology 148, 978–990 (2015).
Chen, C. et al. Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151-5p as a therapeutic target for systemic sclerosis. Cell Res. 27, 559–577 (2017).
Meisel, R. et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103, 4619–4621 (2004).
Raghuvanshi, S., Sharma, P., Singh, S., Van Kaer, L. & Das, G. Mycobacterium tuberculosis evades host immunity by recruiting mesenchymal stem cells. Proc. Natl Acad. Sci. USA 107, 21653–21658 (2010).
Dhingra, S. et al. Preserving prostaglandin E2 level prevents rejection of implanted allogeneic mesenchymal stem cells and restores postinfarction ventricular function. Circulation 128, S69–S78 (2013).
Boumaza, I. et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J. Autoimmun. 32, 33–42 (2009).
Patel, S. A. et al. Role of mesenchymal stem cell-derived cancer cells through regulatory T cells: mesenchymal stem cells protect breast. J. Immunol. 184, 5885–5894 (2010).
Park, H. J., Oh, S. H., Kim, H. N., Jung, Y. J. & Lee, P. H. Mesenchymal stem cells enhance α-synuclein clearance via M2 microglia polarization in experimental and human parkinsonian disorder. Acta Neuropathol. 132, 685–701 (2016).
Lee, K. C., Lin, H. C., Huang, Y. H. & Hung, S. C. Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J. Hepatol. 63, 1405–1412 (2015).
Christiansen, O. B., Steffensen, R., Nielsen, H. S. & Varming, K. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol. Obstet. Invest. 66, 257–267 (2008).
Chavan, A. R., Griffith, O. W. & Wagner, G. P. The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr. Opin. Genet. Dev. 47, 24–32 (2017).
Arck, P. C. & Hecher, K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med. 19, 548–556 (2013).
Kanellopoulos-Langevin, C., Caucheteux, S. M., Verbeke, P. & Ojcius, D. M. Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto-maternal interface. Reprod. Biol. Endocrinol. 1, 121 (2003).
Giakoumelou, S. et al. The role of infection in miscarriage. Hum. Reprod. Update 22, 116–133 (2016).
Girardi, G., Yarilin, D., Thurman, J. M., Holers, V. M. & Salmon, J. E. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 203, 2165–2175 (2006).
Kwak-Kim, J., Bao, S., Lee, S. K., Kim, J. W. & Gilman-Sachs, A. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am. J. Reprod. Immunol. 72, 129–140 (2014).
Luna, R. L. et al. Sildenafil (Viagra ®) blocks inflammatory injury in LPS-induced mouse abortion: a potential prophylactic treatment against acute pregnancy loss? Placenta 36, 1122–1129 (2015).
Lee, A. J., Kandiah, N., Karimi, K., Clark, D. A. & Ashkar, A. A. Interleukin-15 is required for maximal lipopolysaccharide-induced abortion. J. Leukoc. Biol. 93, 905–912 (2013).
Keating, A. Mesenchymal stromal cells: new directions. Cell Stem Cell 10, 709–716 (2012).
Shi, Y. et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 20, 510–518 (2010).
Willis, G. R. et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 197, 104–116 (2018).
Bai, M. et al. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 93, 814–825 (2018).
Zhang, Y. et al. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology 59, 671–682 (2014).
Shi, Y. et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 14, 493–507 (2018).
Galipeau, J. & Sensebe, L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833 (2018).
Watschinger, K. et al. Tetrahydrobiopterin and alkylglycerol monooxygenase substantially alter the murine macrophage lipidome. Proc. Natl Acad. Sci. USA 112, 2431–2436 (2015).
Herd, H. L., Bartlett, K. T., Gustafson, J. A., McGill, L. D. & Ghandehari, H. Macrophage silica nanoparticle response is phenotypically dependent. Biomaterials 53, 574–582 (2015).
Jiang, X., Du, M. R., Li, M. & Wang, H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell. Mol. Immunol. 15, 1027–1037 (2018).
Li Y., Li D., Du M. TIM-3: a crucial regulator of NK cells in pregnancy. Cell. Mol. Immunol. (2017). https://doi.org/10.1038/cmi.2017.85.
Wong, L. F., Porter, T. F. & Scott, J. R. Immunotherapy for recurrent miscarriage. Cochrane Database Syst. Rev. 10, 1–50 (2014).
Singer, N. G. & Caplan, A. I. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 6, 457–478 (2011).
Zhang, Z. et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 27(Suppl 2), 112–120 (2012).
Shi, M. et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl. Med. 1, 725–731 (2012).
Wakitani, S. et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J. Tissue Eng. Regen. Med. 5, 146–150 (2011).
He, Y. et al. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res. Ther. 9, 263 (2018).
Lee, H. J. et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J. Clin. Oncol. 25, 3198–3204 (2007).
Du, H. & Taylor, H. S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25, 2082–2086 (2007).
Du, H., Naqvi, H. & Taylor, H. S. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 21, 3324–3331 (2012).
Mosna, F., Sensebe, L. & Krampera, M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 19, 1449–1470 (2010).
Ko, J. H. et al. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc. Natl Acad. Sci. USA 113, 158–163 (2016).
Prockop, D. J. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells 31, 2042–2046 (2013).
Wang, G. et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 25, 1209–1223 (2018).
Su, J. et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 21, 388–396 (2014).
Lee, R. H. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63 (2009).
Choi, H., Lee, R. H., Bazhanov, N., Oh, J. Y. & Prockop, D. J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118, 330–338 (2011).
Qi, Y. et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin. J. Invest. Dermatol. 134, 526–537 (2014).
Mittal, M. et al. TNFα-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc. Natl Acad. Sci. USA 113, E8151–E8158 (2016).
Song, H. B. et al. Mesenchymal stromal cells inhibit inflammatory lymphangiogenesis in the cornea by suppressing macrophage in a TSG-6-dependent manner. Mol. Ther. 26, 162–172 (2018).
Valles, G. et al. Topographical cues regulate the crosstalk between MSCs and macrophages. Biomaterials 37, 124–133 (2015).
Kota, D. J., Wiggins, L. L., Yoon, N. & Lee, R. H. TSG-6 produced by hMSCs delays the onset of autoimmune diabetes by suppressing Th1 development and enhancing tolerogenicity. Diabetes 62, 2048–2058 (2013).
Espagnolle, N., Balguerie, A., Arnaud, E., Sensebé, L. & Varin, A. CD54-mediated interaction with pro-inflammatory macrophages increases the immunosuppressive function of human mesenchymal stromal cells. Stem Cell Rep. 8, 961–976 (2017).
Amouzegar, A., Mittal, S. K., Sahu, A., Sahu, S. K. & Chauhan, S. K. Mesenchymal stem cells modulate differentiation of myeloid progenitor cells during inflammation. Stem Cells 35, 1532–1541 (2017).
Wisniewski, H. G. & Vilcek, J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 8, 143–156 (1997).
Jenmalm, M. C., Cherwinski, H., Bowman, E. P., Phillips, J. H. & Sedgwick, J. D. Regulation of myeloid cell function through the CD200 receptor. J. Immunol. 176, 191–199 (2006).
Wright, G. J. et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13, 233–242 (2000).
Sheng, H. et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through upregulation of B7-H1. Cell Res. 18, 846–857 (2008).
Acknowledgements
This work was supported by the National Basic Research Program of China (2015CB943300 and 2017YFC1001403), the Nature Science Foundation from the National Nature Science Foundation of China (NSFC) (81630036, 91542116, 31570920, 81490744, 31171437, 31270969, 81571512, and 81501334), the Innovation-oriented Science and Technology Grant from the NHC Key Laboratory of Reproduction Regulation (CX2017-2), the Program of Shanghai Academic/Technology Research Leader (17XD1400900), the Key Project of Shanghai Municipal Education Commission (MECSM) (14ZZ013), and the Key Project of Shanghai Basic Research from Shanghai Municipal Science and Technology Commission (STCSM) (12JC1401600).
Author contributions
L.Y.H., experimental design, performance of experiments and data analysis, and manuscript writing; Z.D. and X.L., performance of experiments, analysis of data, and generation of figures; Z.J., L.Y.K. and H.J.F., generation of figures and literature search; Z.Y.Y., Z.X.X. and L.D.J., study design and data interpretation; D.M.R., study design, conception of experiments and data interpretation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, D., Xu, L. et al. Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol 16, 908–920 (2019). https://doi.org/10.1038/s41423-019-0204-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0204-6
This article is cited by
-
Neu5Gc regulates decidual macrophages leading to abnormal embryo implantation
Genes & Immunity (2024)
-
Decidual macrophage: a reversible role in immunotolerance between mother and fetus during pregnancy
Archives of Gynecology and Obstetrics (2024)
-
Adipose Tissue and Bone Marrow-Derived Mesenchymal Stem Cells are not Really the Same: Investigating the Differences in Their Immunomodulatory, Migratory, and Adhesive Profile
Biochemical Genetics (2024)
-
Human mesenchymal stem cells derived from adipose tissue showed a more robust effect than those from the umbilical cord in promoting corneal graft survival by suppressing lymphangiogenesis
Stem Cell Research & Therapy (2023)
-
PD-L1 expression levels in mesenchymal stromal cells predict their therapeutic values for autoimmune hepatitis
Stem Cell Research & Therapy (2023)