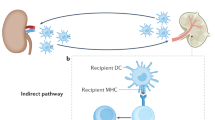
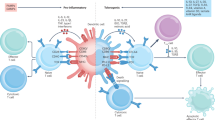
Abstract
Donor-specific transplantation tolerance that enables weaning from immunosuppressive drugs but retains immune competence to non-graft antigens has been a lasting pursuit since the discovery of neonatal tolerance. More recently, efforts have been devoted not only to understanding how transplantation tolerance can be induced but also the mechanisms necessary to maintain it as well as how inflammatory exposure challenges its durability. This review focuses on recent advances regarding key peripheral mechanisms of T cell tolerance, with the underlying hypothesis that a combination of several of these mechanisms may afford a more robust and durable tolerance and that a better understanding of these individual pathways may permit longitudinal tracking of tolerance following clinical transplantation to serve as biomarkers. This review may enable a personalized assessment of the degree of tolerance in individual patients and the opportunity to strengthen the robustness of peripheral tolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Brouard, S. et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am. J. Transplant. 12, 3296–3307 (2012).
Kawai, T. et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 358, 353–361 (2008).
Scandling, J. D. et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 358, 362–368 (2008).
Leventhal, J. et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci. Transl. Med 4, 124ra128 (2012).
Kawai, T. et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am. J. Transplant. 14, 1599–1611 (2014).
Miller, M. L., Chong, A. S. & Alegre, M. L. Fifty shades of tolerance. Curr. Transplant. Rep. 4, 262–269 (2017).
You S. and Chatenoud L. The concerted action of multiple mechanisms to induce and sustain transplant tolerance. OBM Transplantation 2, (2018) https://doi.org/10.21926/obm.transplant.1804025.
Wang, T. et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am. J. Transplant. 10, 1524–1533 (2010).
Kawai, T. et al. Tolerance: one transplant for life. Transplantation 98, 117–121 (2014).
Nikolich-Zugich, J., Slifka, M. K. & Messaoudi, I. The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol. 4, 123–132 (2004).
Honjo, K., Xu, X. Y. & Bucy, R. P. Heterogeneity of T cell clones specific for a single indirect alloantigenic epitope (I-Ab/H-2Kd54-68) that mediate transplant rejection. Transplantation 70, 1516–1524 (2000).
Zhong, S. et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc. Natl Acad. Sci. USA 110, 6973–6978 (2013).
Krogsgaard, M. et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol. Cell 12, 1367–1378 (2003).
Labrecque, N. et al. How much TCR does a T cell need? Immunity 15, 71–82 (2001).
Cawthon, A. G., Lu, H. & Alexander-Miller, M. A. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8αβ versus CD8αα expression. J. Immunol. 167, 2577–2584 (2001).
Kuball, J. et al. Increasing functional avidity of TCR-redirected T cells by removing defined glycosylation sites in the TCR constant domain. J. Exp. Med. 206, 463–475 (2009).
Minguet, S., Swamy, M., Alarcón, B., Luescher, I. F. & Schamel, W. W. A. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity 26, 43–54 (2007).
Fahmy, T. M., Bieler, J. G., Edidin, M. & Schneck, J. P. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity 14, 135–143 (2001).
Richer, M. J., Nolz, J. C. & Harty, J. T. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity 38, 140–152 (2013).
Persaud, S. P., Parker, C. R., Lo, W.-L., Weber, K. S. & Allen, P. M. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat. Immunol. 15, 266–274 (2014).
Ioannidou, K. et al. Heterogeneity assessment of functional T cell avidity. Sci. Rep. 7, 44320–44320 (2017).
von Essen, M. R., Kongsbak, M. & Geisler, C. Mechanisms behind functional avidity maturation in T cells. Clin. Dev. Immunol. 2012, 163453 (2012).
Hesse, M. D., Karulin, A. Y., Boehm, B. O., Lehmann, P. V. & Tary-Lehmann, M. A. T. Cell clone’s avidity is a function of its activation state. J. Immunol. 167, 1353–1361 (2001).
Miller, M. L. et al. Distinct graft-specific TCR avidity profiles during acute rejection and tolerance. Cell Rep. 24, 2112–2126 (2018).
Savage, P. A., Boniface, J. J. & Davis, M. M. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10, 485–492 (1999).
Slifka, M. K. & Whitton, J. L. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat. Immunol. 2, 711–717 (2001).
Busch, D. H. & Pamer, E. G. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189, 701–710 (1999).
Dutoit, V. et al. Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 61, 5850–5856 (2001).
Honjo, K., Yan, Xu. X., Kapp, J. A. & Bucy, R. P. Evidence for cooperativity in the rejection of cardiac grafts mediated by CD4+ TCR Tg T cells specific for a defined allopeptide. Am. J. Transplant. 4, 1762–1768 (2004).
Enouz, S., Carrie, L., Merkler, D., Bevan, M. J. & Zehn, D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J. Exp. Med. 209, 1769–1779 (2012).
Ozga, A. J. et al. pMHC affinity controls duration of CD8+ T cell–DC interactions and imprints timing of effector differentiation versus expansion. J. Exp. Med. 213, 2811–2829 (2016).
Alexander-Miller, M. A., Leggatt, G. R. & Berzofsky, J. A. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. 93, 4102–4107 (1996).
Zeh, H. J., Perry-Lalley, D., Dudley, M. E., Rosenberg, S. A. & Yang, J. C. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 162, 989–994 (1999).
Zhu, Z. et al. CD4+ T cell help selectively enhances high-avidity tumor antigen-specific CD8+ T Cells. J. Immunol. 195, 3482–3489 (2015).
Zehn, D. & Bevan, M. J. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity 25, 261–270 (2006).
Anderton, S. M. & Fillatreau, S. Activated B cells in autoimmune diseases: the case for a regulatory role. Nat. Clin. Pract. Rheumatol. 4, 657–666 (2008).
Black, C. M., Armstrong, T. D. & Jaffee, E. M. Apoptosis-regulated low-avidity cancer-specific CD8(+) T cells can be rescued to eliminate HER2/neu-expressing tumors by costimulatory agonists in tolerized mice. Cancer Immunol. Res 2, 307–319 (2014).
Mallone, R. et al. Functional avidity directs T-cell fate in autoreactive CD4+ T cells. Blood 106, 2798–2805 (2005).
Yeh, W. I. et al. Avidity and bystander suppressive capacity of human regulatory T cells expressing de novo autoreactive t-cell receptors in Type 1 diabetes. Front. Immunol. 8, 1313 (2017).
Tsang, J. Y. et al. The potency of allospecific Tregs cells appears to correlate with T cell receptor functional avidity. Am. J. Transplant. 11, 1610–1620 (2011).
Lee, I. et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 201, 1037–1044 (2005).
Young, J. S. et al. Erosion of transplantation tolerance after infection. Am. J. Transplant. 17, 81–90 (2017).
Francis, R. S. et al. Induction of transplantation tolerance converts potential effector T cells into graft-protective regulatory T cells. Eur. J. Immunol. 41, 726–738 (2011).
Kendal, A. R. et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J. Exp. Med. 208, 2043–2053 (2011).
Brennan, T. V. et al. Requirements for prolongation of allograft survival with regulatory T cell infusion in lymphosufficient hosts. J. Surg. Res. 169, e69–e75 (2011).
Lin, C. Y., Graca, L., Cobbold, S. P. & Waldmann, H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat. Immunol. 3, 1208–1213 (2002).
Graca, L. et al. Both CD4(+)CD25(+) and CD4(+)CD25(−) regulatory cells mediate dominant transplantation tolerance. J. Immunol. 168, 5558–5565 (2002).
Feng, G. et al. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3 + regulatory T cells. Eur. J. Immunol. 38, 2512–2527 (2008).
Graca, L., Cobbold, S. P. & Waldmann, H. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 195, 1641–1646 (2002).
Chen, L. et al. TLR engagement prevents transplantation tolerance. Am. J. Transplant. 6, 2282–2291 (2006).
Miller, M. L. et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat. Commun. 6, 7566 (2015).
Fan, Z. et al. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat. Med. 16, 718–722 (2010).
Regateiro, F. S. et al. Foxp3 expression is required for the induction of therapeutic tissue tolerance. J. Immunol. 189, 3947–3956 (2012).
Yamaguchi, T., Wing, J. B. & Sakaguchi, S. Two modes of immune suppression by Foxp3( ) regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 23, 424–430 (2011).
Thornton, A. M. & Shevach, E. M. CD4 + CD25 + immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188, 287–296 (1998).
Fallarino, F. et al. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4, 1206–1212 (2003).
Chen, W., Liang, X., Peterson, A. J., Munn, D. H. & Blazar, B. R. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J. Immunol. 181, 5396–5404 (2008).
Wang, J. et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell 176, 1–14 (2019).
Collison, L. W. et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 (2007).
Huang, C. T. et al. Role of LAG-3 in regulatory T cells. Immunity 21, 503–513 (2004).
Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532 (2008).
Cretney, E., Kallies, A. & Nutt, S. L. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 34, 74–80 (2013).
Zheng, Y. et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458, 351–356 (2009).
Koch, M. A. et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009).
Levine, A. G. et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 546, 421–425 (2017).
Ohnmacht, C. et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349, 989–993 (2015).
Sefik, E. et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349, 993–997 (2015).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17, 983–988 (2011).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Yang, S., Fujikado, N., Kolodin, D., Benoist, C. & Mathis, D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348, 589–594 (2015).
Malchow, S. et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44, 1102–1113 (2016).
Kieback, E. et al. Thymus-derived regulatory T cells are positively selected on natural self-antigen through cognate interactions of high functional avidity. Immunity 44, 1114–1126 (2016).
Legoux, F. P. et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity 43, 896–908 (2015).
Aschenbrenner, K. et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire + medullary thymic epithelial cells. Nat. Immunol. 8, 351–358 (2007).
Malhotra, D. et al. Tolerance is established in polyclonal CD4( + ) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat. Immunol. 17, 187–195 (2016).
LeGuern, C. & Germana, S. On the elusive TCR specificity of thymic regulatory T cells. Am. J. Transplant. 19, 15–20 (2019).
Kanamori, M., Nakatsukasa, H., Okada, M., Lu, Q. & Yoshimura, A. Induced regulatory T cells: their development, stability, and applications. Trends Immunol. 37, 803–811 (2016).
Bailey-Bucktrout, S. L. et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39, 949–962 (2013).
Ezzelarab, M. B. et al. Regulatory T cell infusion can enhance memory T cell and alloantibody responses in lymphodepleted nonhuman primate heart allograft recipients. Am. J. Transplant. 16, 1999–2015 (2016).
Sakaguchi, S., Vignali, D. A., Rudensky, A. Y., Niec, R. E. & Waldmann, H. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13, 461–467 (2013).
Graca, L. et al. Donor-specific transplantation tolerance: the paradoxical behavior of CD4+CD25+ T cells. Proc. Natl Acad. Sci. USA 101, 10122–10126 (2004).
Sagoo, P. et al. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med 3, 83ra42 (2011).
Carvalho-Gaspar, M. et al. Location and time-dependent control of rejection by regulatory T cells culminates in a failure to generate memory T cells. J. Immunol. 180, 6640–6648 (2008).
Golshayan, D. et al. In vitro-expanded donor alloantigen-specific CD4+ CD25 + regulatory T cells promote experimental transplantation tolerance. Blood 109, 827–835 (2007).
Joffre, O. et al. Prevention of acute and chronic allograft rejection with CD4 + CD25 + Foxp3 + regulatory T lymphocytes. Nat. Med. 14, 88–92 (2008).
MacDonald, K. G. et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 126, 1413–1424 (2016).
Moore, C. et al. Alloreactive regulatory T cells generated with retinoic acid prevent skin allograft rejection. Eur. J. Immunol. 45, 452–463 (2015).
Sanchez-Fueyo, A. et al. Specificity of CD4+CD25 + regulatory T cell function in alloimmunity. J. Immunol. 176, 329–334 (2006).
Tsang, J. Y. et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J. Clin. Invest. 118, 3619–3628 (2008).
Noyan, F. et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am. J. Transplant. 17, 917–930 (2017).
Boardman, D. A. et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transplant. 17, 931–943 (2017).
Pierini, A. et al. T cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight 2, 92865 (2017).
Zhang, Q. et al. Chimeric Antigen Receptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance. Front. Immunol. 9, 2359 (2018).
Veerapathran, A., Pidala, J., Beato, F., Yu, X. Z. & Anasetti, C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood 118, 5671–5680 (2011).
Tang, Q. & Vincenti, F. Transplant trials with Tregs: perils and promises. J. Clin. Invest. 127, 2505–2512 (2017).
Young, J. S., Yin, D., Vannier, A. G. L., Alegre, M. L. & Chong, A. S. Equal expansion of endogenous transplant-specific regulatory T cell and recruitment into the allograft during rejection and tolerance. Front. Immunol. 9, 1385 (2018).
Tang, Q. & Lee, K. Regulatory T-cell therapy for transplantation: how many cells do we need? Curr. Opin. Organ Transplant. 17, 349–354 (2012).
Hansen, W. et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 209, 2001–2016 (2012).
Delgoffe, G. M. et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 (2013).
Glinka, Y. & Prud’homme, G. J. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 84, 302–310 (2008).
Bono, M. R., Fernandez, D., Flores-Santibanez, F., Rosemblatt, M. & Sauma, D. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 589, 3454–3460 (2015).
Jin, D. et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 70, 2245–2255 (2010).
Stolp, J., Turka, L. A. & Wood, K. J. B cells with immune-regulating function in transplantation. Nat. Rev. Nephrol. 10, 389–397 (2014).
Fillatreau, S. Regulatory plasma cells. Curr. Opin. Pharmacol. 23, 1–5 (2015).
Shen, P. et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370 (2014).
Lino, A. C. et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity 49, 120–133 (2018).
Xiao, S., Brooks, C. R., Sobel, R. A. & Kuchroo, V. K. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J. Immunol. 194, 1602–1608 (2015).
Yeung, M. Y. et al. TIM-1 signaling is required for maintenance and induction of regulatory B cells. Am. J. Transplant. 15, 942–953 (2015).
Chihara, N. et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 558, 454–459 (2018).
Yan, Y. et al. Postoperative administration of donor B cells induces rat kidney allograft acceptance: lack of association with Th2 cytokine expression in long-term accepted grafts. Transplantation 73, 1123–1130 (2002).
Fehr, T. et al. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells, and MHC class II. J. Immunol. 181, 165–173 (2008).
Deng, S. et al. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J. Immunol. 178, 6028–6032 (2007).
Ding, Q. et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Invest. 121, 3645–3656 (2011).
Lee, K. M. et al. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am. J. Transplant. 12, 2072–2078 (2012).
Sun, J. et al. Transcriptomics identify CD9 as a marker of murine IL-10-competent regulatory B cells. Cell Rep. 13, 1110–1117 (2015).
Clatworthy, M. R. et al. B-cell-depleting induction therapy and acute cellular rejection. N. Engl. J. Med. 360, 2683–2685 (2009).
Pallier, A. et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 78, 503–513 (2010).
Sagoo, P. et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J. Clin. Invest. 120, 1848–1861 (2010).
Newell, K. A. et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J. Clin. Invest. 120, 1836–1847 (2010).
Nova-Lamperti, E. et al. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4 + T-cell responses. Sci. Rep. 6, 20044 (2016).
Chesneau, M. et al. Tolerant kidney transplant patients produce B Cells with regulatory properties. J. Am. Soc. Nephrol. 26, 2588–2598 (2015).
Rebollo-Mesa, I. et al. Biomarkers of tolerance in kidney transplantation: are we predicting tolerance or response to immunosuppressive treatment? Am. J. Transplant. 16, 3443–3457 (2016).
Bottomley, M. J., Chen, M., Fuggle, S., Harden, P. N. & Wood, K. J. Application of operational tolerance signatures are limited by variability and type of immunosuppression in renal transplant recipients: a Cross-Sectional Study. Transplant. Direct 3, e125 (2017).
Schietinger, A. & Greenberg, P. D. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 35, 51–60 (2014).
Thommen, D. S. & Schumacher, T. N. T Cell Dysfunction in Cancer. Cancer Cell 33, 547–562 (2018).
Hashimoto, M. et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu. Rev. Med. 69, 301–318 (2018).
Wang, C., Singer, M. & Anderson, A. C. Molecular Dissection of CD8(+) T-Cell Dysfunction. Trends Immunol. 38, 567–576 (2017).
Oxenius, A., Zinkernagel, R. M. & Hengartner, H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9, 449–457 (1998).
Ciurea, A., Hunziker, L., Klenerman, P., Hengartner, H. & Zinkernagel, R. M. Impairment of CD4(+) T cell responses during chronic virus infection prevents neutralizing antibody responses against virus escape mutants. J. Exp. Med. 193, 297–305 (2001).
Fuller, M. J. & Zajac, A. J. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170, 477–486 (2003).
Brooks, D. G., Teyton, L., Oldstone, M. B. & McGavern, D. B. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 79, 10514–10527 (2005).
Crawford, A. et al. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014).
Ejrnaes, M. et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203, 2461–2472 (2006).
Elsaesser, H., Sauer, K. & Brooks, D. G. IL-21 is required to control chronic viral infection. Science 324, 1569–1572 (2009).
Quezada, S. A., Jarvinen, L. Z., Lind, E. F. & Noelle, R. J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22, 307–328 (2004).
Baas, M. et al. TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. eLife 5, e08133 (2016).
Besancon, A. et al. The induction and maintenance of transplant tolerance engages both regulatory and anergic CD4(+) T cells. Front. Immunol. 8, 218 (2017).
Quezada, S. A. et al. Mechanisms of donor-specific transfusion tolerance: preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood 102, 1920–1926 (2003).
Chai, J. G. et al. Allospecific CD4(+) T cells retain effector function and are actively regulated by Treg cells in the context of transplantation tolerance. Eur. J. Immunol. 45, 2017–2027 (2015).
Ferrer, I. R. et al. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc. Natl. Acad. Sci. USA 108, 20701–20706 (2011).
Jiang, X. et al. Cardiac allograft acceptance induced by blockade of CD40-CD40L costimulation is dependent on CD4+ CD25+ regulatory T cells. Surgery 149, 336–346 (2011).
Wu, J. et al. Ablation of Transcription Factor IRF4 Promotes Transplant Acceptance by Driving Allogenic CD4(+) T Cell Dysfunction. Immunity 47, 1114–1128 (2017).
Miller, M. L. et al. Tracking of TCR-transgenic T cells reveals that multiple mechanisms maintain cardiac transplant tolerance in mice. Am. J. Transplant. 16, 2854–2864 (2016).
Man, K. et al. Transcription factor IRF4 promotes CD8(+) T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity 47, 1129–1141 (2017).
Zhang, H. et al. Ablation of interferon regulatory factor 4 in T cells induces “memory” of transplant tolerance that is irreversible by immune checkpoint blockade. Am. J. Transplant. 3, 1–10 (2018).
Sarraj, B. et al. Impaired selectin-dependent leukocyte recruitment induces T-cell exhaustion and prevents chronic allograft vasculopathy and rejection. Proc. Natl Acad. Sci. USA 111, 12145–12150 (2014).
Kalekar, L. A. et al. CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat. Immunol. 17, 304–314 (2016).
Paley, M. A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008).
He, R. et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 537, 412–428 (2016).
Im, S. J. et al. Defining CD8+T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Singer, M. et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell 166, 1500–1511 (2016).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Jayachandran, R. et al. Disruption of Coronin 1 signaling in T cells promotes allograft tolerance while maintaining anti-pathogen immunity. Immunity 50, 152–165 (2019).
Yang, J. et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl Acad. Sci. USA 104, 19954–19959 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gupta, P.K., McIntosh, C.M., Chong, A.S. et al. The pursuit of transplantation tolerance: new mechanistic insights. Cell Mol Immunol 16, 324–333 (2019). https://doi.org/10.1038/s41423-019-0203-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0203-7
Keywords
This article is cited by
-
Maternal dendritic cells influence fetal allograft response following murine in-utero hematopoietic stem cell transplantation
Stem Cell Research & Therapy (2023)
-
Loss of Lkb1 impairs Treg function and stability to aggravate graft-versus-host disease after bone marrow transplantation
Cellular & Molecular Immunology (2020)