Abstract
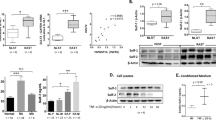
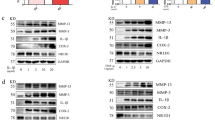
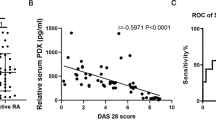
Cystathionine-γ-lyase (CSE), an enzyme associated with hydrogen sulfide (H2S) production, is an important endogenous regulator of inflammation. Jumonji domain-containing protein 3 (JMJD3) is implicated in the immune response and inflammation. Here, we investigated the potential contribution of JMJD3 to endogenous CSE-mediated inflammation in rheumatoid arthritis (RA). Upregulated CSE and JMJD3 were identified in synovial fibroblasts (SFs) from RA patients as well as in the joints of arthritic mice. Knocking down CSE augmented inflammation in IL-1β-induced SFs by increasing JMJD3 expression. In addition, CSE−/− mice with collagen-induced arthritis (CIA) developed severe joint inflammation and bone erosion. Conversely, overexpressing CSE inhibited JMJD3 expression by the transcription factor Sp-1 and was accompanied by reduced inflammation in IL-1β-treated SFs. Furthermore, JMJD3 silencing or the administration of the JMJD3 inhibitor GSK-J4 significantly decreased the inflammatory response in IL-1β-treated SFs, mainly by controlling the methylation status of H3K27me3 at the promoter of its target genes. GSK-J4 markedly attenuated the severity of arthritis in CIA mice. In conclusion, suppressing JMJD3 expression by the transcription factor Sp-1 is likely responsible for the ability of CSE to negatively modulate the inflammatory response and reduce the progression of RA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Walsh, D. A. & McWilliams, D. F. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 581–592 (2014).
Anderson, K. O., Bradley, L. A., Young, L. D., McDaniel, L. K. & Wise, C. M. Rheumatoid arthritis: review of psychological factors related to etiology, effects, and treatment. Psychol. Bull. 98, 358–387 (1985).
Choy, E. H. & Panayi, G. S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 344, 907–916 (2001).
Wang L. et al. TXNDC5 synergizes with HSC70 to exacerbate the inflammatory phenotype of synovial fibroblasts in rheumatoid arthritis through NF-kappaB signaling. Cell Mol Immunol. 4, 1–12 (2017).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann. Rheum. Dis. 73, 492–509 (2014).
Zhou, H. F. et al. Peptide-siRNA nanocomplexes targeting NF-kappaB subunit p65 suppress nascent experimental arthritis. J. Clin. Invest. 124, 4363–4374 (2014).
Salliot, C. et al. Infections during tumour necrosis factor-alpha blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatol. (Oxf.) 46, 327–334 (2007).
Emery, P. et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 67, 1516–1523 (2008).
Medzhitov, R. & Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 9, 692–703 (2009).
Han, X. et al. Epigenetic regulation of tumor necrosis factor alpha (TNFalpha) release in human macrophages by HIV-1 single-stranded RNA (ssRNA) is dependent on TLR8 signaling. J. Biol. Chem. 287, 13778–13786 (2012).
Das, N. D. et al. Gene networking and inflammatory pathway analysis in a JMJD3 knockdown human monocytic cell line. Cell Biochem. Funct. 30, 224–232 (2012).
Cao, R. et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 (2002).
Ekundi-Valentim, E. et al. Differing effects of exogenous and endogenous hydrogen sulphide in carrageenan-induced knee joint synovitis in the rat. Br. J. Pharmacol. 159, 1463–1474 (2010).
Wallace, J. L. & Wang, R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug. Discov. 14, 329–345 (2015).
Stipanuk, M. H. & Beck, P. W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 206, 267–277 (1982).
Awata, S., Nakayama, K., Suzuki, I., Sugahara, K. & Kodama, H. Changes in cystathionine gamma-lyase in various regions of rat brain during development. Biochem. Mol. Biol. Int. 35, 1331–1338 (1995).
Zanardo, R. C. et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 20, 2118–2120 (2006).
Cunha, T. M. et al. Dual role of hydrogen sulfide in mechanical inflammatory hypernociception. Eur. J. Pharmacol. 590, 127–135 (2008).
Oh, G. S. et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 41, 106–119 (2006).
Wu, W. J. et al. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 10, 157–167 (2016).
Impellizzeri, D. et al. Oleuropein aglycone, an olive oil compound, ameliorates development of arthritis caused by injection of collagen type II in mice. J. Pharmacol. Exp. Ther. 339, 859–869 (2011).
Neidhart, M., Gay, R. E. & Gay, S. Anti-interleukin-1 and anti-CD44 interventions producing significant inhibition of cartilage destruction in an in vitro model of cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 43, 1719–1728 (2000).
Mazess, R. B., Nord, R., Hanson, J. A. & Barden, H. S. Bilateral measurement of femoral bone mineral density. J. Clin. Densitom. 3, 133–140 (2000).
Kawai, M. et al. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc. Natl. Acad. Sci. USA 107, 10508–10513 (2010).
Mukaida, N., Mahe, Y. & Matsushima, K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 265, 21128–21133 (1990).
Hashizume, R. et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat. Med. 20, 1394–1396 (2014).
Yasui, T. et al. Epigenetic regulation of osteoclast differentiation: possible involvement of Jmjd3 in the histone demethylation of Nfatc1. J. Bone Miner. Res. 26, 2665–2671 (2011).
Jia W. et al. Histone demethylase JMJD3 regulates fibroblast-like synoviocyte-mediated proliferation and joint destruction in rheumatoid arthritis. FASEB J. 2018; fj201701483R.
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Bartok, B. & Firestein, G. S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255 (2010).
Guo N. et al. A critical epitope in CD147 facilitates memory CD4(+) T-cell hyper-activation in rheumatoid arthritis. Cell Mol. Immunol. 2018; in press. https://doi.org/10.1038/s41423-018-0012-4.
Leech, M. T. & Morand, E. F. Fibroblasts and synovial immunity. Curr. Opin. Pharmacol. 13, 565–569 (2013).
Li, T. et al. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp. Biol. Med. (Maywood). 233, 1081–1087 (2008).
Zhang, H. & Bhatia, M. Hydrogen sulfide: a novel mediator of leukocyte activation. Immunopharmacol. Immunotoxicol. 30, 631–645 (2008).
Wallace, J. L., Caliendo, G., Santagada, V., Cirino, G. & Fiorucci, S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 132, 261–271 (2007).
Choi, H. M. et al. Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Res. Ther. 11, R161 (2009).
Gong, Q. H. et al. Hydrogen sulfide attenuates lipopolysaccharide-induced cognitive impairment: a pro-inflammatory pathway in rats. Pharmacol. Biochem. Behav. 96, 52–58 (2010).
Zhang, Q. et al. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol. Res. 84, 32–44 (2014).
Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 90, 9–17 (2011).
Swigut, T. & Wysocka, J. H3K27 demethylases, at long last. Cell 131, 29–32 (2007).
Zhang, X., Li, L., Fourie, J., Davie, J. R. & Guarcello VDiasio, R. B. The role of Sp1 and Sp3 in the constitutive DPYD gene expression. Biochim. Biophys. Acta 1759, 247–256 (2006).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Joosten, L. A. et al. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J. Immunol. 171, 6145–6153 (2003).
De Santa, F. et al. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 130, 1083–1094 (2007).
Kruidenier, L. et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 488, 404–408 (2012).
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81673428; 81330080), a key laboratory program of the Education Commission of Shanghai Municipality (No. ZDSYS14005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wu, W., Qin, M., Jia, W. et al. Cystathionine-γ-lyase ameliorates the histone demethylase JMJD3-mediated autoimmune response in rheumatoid arthritis. Cell Mol Immunol 16, 694–705 (2019). https://doi.org/10.1038/s41423-018-0037-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-018-0037-8
Keywords
This article is cited by
-
YAP represses intestinal inflammation through epigenetic silencing of JMJD3
Clinical Epigenetics (2024)
-
Innate immune memory in inflammatory arthritis
Nature Reviews Rheumatology (2023)
-
Histone demethylases in the regulation of immunity and inflammation
Cell Death Discovery (2023)
-
JMJD3 ablation in myeloid cells confers renoprotection in mice with DOCA/salt-induced hypertension
Hypertension Research (2023)
-
Signaling pathways in rheumatoid arthritis: implications for targeted therapy
Signal Transduction and Targeted Therapy (2023)