Abstract
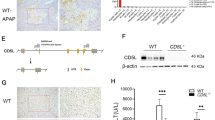
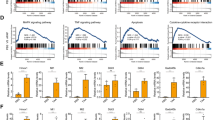
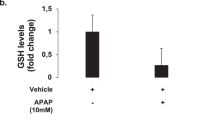
Induction of osteopontin (OPN), a well-known pro-inflammatory molecule, has been observed in acetaminophen (APAP)-induced hepatotoxicity. However, the precise cell source for OPN induction and its role during APAP-induced hepatotoxicity has not been fully explored. By employing a hepatotoxic mouse model induced by APAP overdose, we demonstrate that both serum and hepatic OPN levels were significantly elevated in response to APAP treatment. Our in vivo and in vitro studies clearly indicated that the induced expression of hepatic OPN was mainly located in necrosis areas and produced by dying or dead hepatocytes. Functional experiments showed that OPN deficiency protected against the APAP-induced liver injury by inhibiting the toxic APAP metabolism via reducing the expression of the cytochrome P450 family 2 subfamily E member 1 (CYP2E1). Interestingly, this inhibition of CYP2E1 expression did not occur in unfasted Opn−/− mice, but was significant in fasted Opn−/− mice and maintained for 2 hours after APAP challenge in fasted Opn−/− mice. In addition, despite the early protective role of OPN deficiency on APAP-induced hepatotoxicity, OPN deficiency retarded injury resolution by sensitizing hepatocytes to apoptosis and impairing liver regeneration. Finally, we demonstrated that a siRNA-mediated transient hepatic Opn knockdown could sufficiently and significantly protect animals from APAP-induced hepatotoxicity and death. In conclusion, this study clearly defines the cell source of OPN induction in response to APAP treatment, provides a novel insight into the metabolic role of OPN to APAP overdose, and suggests an Opn-targeted therapeutic strategy for the treatment or prevention of APAP-induced hepatotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Davidson, D. G. & Eastham, W. N. Acute liver necrosis following overdose of paracetamol. Br. Med. J. 2, 497–499 (1966).
Stravitz, R. T. & Kramer, D. J. Management of acute liver failure. Nat. Rev. Gastroenterol. Hepatol. 6, 542–553 (2009).
Larson, A. M. et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42, 1364–1372 (2005).
Dahlin, D. C., Miwa, G. T., Lu, A. Y. & Nelson, S. D. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl Acad. Sci. USA 81, 1327–1331 (1984).
Lee, S. S., Buters, J. T., Pineau, T., Fernandez-Salguero, P. & Gonzalez, F. J. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 271, 12063–12067 (1996).
Novak, R. F. & Woodcroft, K. J. The alcohol-inducible form of cytochrome P450 (CYP 2E1): role in toxicology and regulation of expression. Arch. Pharmacol. Res. 23, 267–282 (2000).
Xie, Y. et al. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 279, 266–274 (2014).
McGill, M. R. et al. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 122, 1574–1583 (2012).
Krenkel, O., Mossanen, J. C. & Tacke, F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg. Nutr. 3, 331–343 (2014).
Lancaster, E. M., Hiatt, J. R. & Zarrinpar, A. Acetaminophen hepatotoxicity: an updated review. Arch. Toxicol. 89, 193–199 (2015).
Jaeschke, H., Williams, C. D., Ramachandran, A. & Bajt, M. L. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 32, 8–20 (2012).
Zhang, C. et al. Macrophage-derived IL-1α promotes sterile inflammation in a mouse model of acetaminophen hepatotoxicity. Cell. Mol. Immunol. (2017). PMID 28504245 (DOI:10.1038/cmi.2017.22). Published online on 15 May, 2017.
Jaeschke, H. Mechanisms of sterile inflammation in acetaminophen hepatotoxicity. Cell. Mol. Immunol. 15, 74–75 (2018).
Iracheta-Vellve, A. & Szabo, G. IL-1α in acetaminophen toxicity: a sterile danger signal. Cell. Mol. Immunol. 15, 284–285 (2018).
Whitcomb, D. C. & Block, G. D. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 272, 1845–1850 (1994).
Bai, J. Adenovirus-mediated expression of CYP2E1 produces liver toxicity in mice. Toxicol. Sci. 91, 365–371 (2006).
Wen, Y., Jeong, S., Xia, Q. & Kong, X. Role of osteopontin in liver diseases. Int. J. Biol. Sci. 12, 1121–1128 (2016).
Kawashima, R. et al. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem. Biophys. Res. Commun. 256, 527–531 (1999).
Fan, X. et al. Intracellular osteopontin inhibits toll-like receptor signaling and impedes liver carcinogenesis. Cancer Res. 75, 86–97 (2015).
Patouraux, S. et al. Osteopontin deficiency aggravates hepatic injury induced by ischemia–reperfusion in mice. Cell Death Dis. 5, e1208 (2014).
Wen, Y. et al. Defective initiation of liver regeneration in osteopontin-deficient mice after partial hepatectomy due to insufficient activation of IL-6/Stat3 pathway. Int. J. Biol. Sci. 11, 1236–1247 (2015).
He, C. Y. et al. The dual role of osteopontin in acetaminophen hepatotoxicity. Acta Pharmacol. Sin. 33, 1004–1012 (2012).
Srungaram, P. et al. Plasma osteopontin in acute liver failure. Cytokine 73, 270–276 (2015).
Saito, Y. et al. Osteopontin small interfering RNA protects mice from fulminant hepatitis. Human. Gene Ther. 18, 1205–1214 (2007).
Wang, Z., Bishop, E. P. & Burke, P. A. Expression profile analysis of the inflammatory response regulated by hepatocyte nuclear factor 4alpha. BMC Genomics 12, 128 (2011).
Hanawa, N. et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 283, 13565–13577 (2008).
Seki, E., Brenner, D. A. & Karin, M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143, 307–320 (2012).
Hu, J., Ramshesh, V. K., McGill, M. R., Jaeschke, H. & Lemasters, J. J. Low dose acetaminophen induces reversible mitochondrial dysfunction associated with transient c-Jun N-terminal kinase activation in mouse liver. Toxicol. Sci. 150, 204–215 (2016).
Ni, H. M., Bockus, A., Boggess, N., Jaeschke, H. & Ding, W. X. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 55, 222–232 (2012).
Lee, J., Giordano, S. & Zhang, J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 441, 523–540 (2012).
Bellward, G. D. et al. Hepatic cytochrome P-450j induction in the spontaneously diabetic BB rat. Mol. Pharmacol. 33, 140–143 (1988).
Song, B. J., Veech, R. L., Park, S. S., Gelboin, H. V. & Gonzalez, F. J. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J. Biol. Chem. 264, 3568–3572 (1989).
Possamai, L. A. et al. Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure*. Crit. Care Med. 41, 2543–2550 (2013).
Yang, M. et al. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol. Lett. 224, 186–195 (2014).
Wang, Y. et al. Increased expression of osteopontin in activated Kupffer cells and hepatic macrophages during macrophage migration in Propionibacterium acnes-treated rat liver. J. Gastroenterol. 35, 696–701 (2000).
Sahai, A. et al. Roles of phosphatidylinositol 3-kinase and osteopontin in steatosis and aminotransferase release by hepatocytes treated with methionine-choline-deficient medium. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G55–G62 (2006).
Wang, X. et al. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut 63, 1805–1818 (2014).
Williams, C. D. et al. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol. Appl. Pharmacol. 275, 122–133 (2014).
Morales-Ibanez, O. et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology 58, 1742–1756 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81670562 and 31300742 to X.K., 81670598 to Q.X., 81372233 to H.W., and 81673935 to X.S.) and a grant from the Shanghai Municipal Education Commission (Gaofeng Clinical Medicine Grant Support (20171911) to X.K., National Science and Technology major grant (2017ZX10203204-006-005) to X.K., and the Shanghai Health Bureau Key Joint Efforts Foundation (2013ZYJB001) to Q.X.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wen, Y., Wang, C., Gu, J. et al. Metabolic modulation of acetaminophen-induced hepatotoxicity by osteopontin. Cell Mol Immunol 16, 483–494 (2019). https://doi.org/10.1038/s41423-018-0033-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-018-0033-z
Keywords
This article is cited by
-
P2rx1 deficiency alleviates acetaminophen-induced acute liver failure by regulating the STING signaling pathway
Cell Biology and Toxicology (2023)
-
The immunological mechanisms and therapeutic potential in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity
Cell & Bioscience (2022)