Abstract
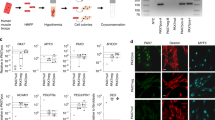
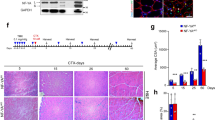
During homeostasis and after injury, adult muscle stem cells (MuSCs) activate to mediate muscle regeneration. However, much remains unclear regarding the heterogeneous capacity of MuSCs for self-renewal and regeneration. Here, we show that Lin28a is expressed in embryonic limb bud muscle progenitors, and that a rare reserve subset of Lin28a+Pax7– skeletal MuSCs can respond to injury at adult stage by replenishing the Pax7+ MuSC pool to drive muscle regeneration. Compared with adult Pax7+ MuSCs, Lin28a+ MuSCs displayed enhanced myogenic potency in vitro and in vivo upon transplantation. The epigenome of adult Lin28a+ MuSCs showed resemblance to embryonic muscle progenitors. In addition, RNA-sequencing revealed that Lin28a+ MuSCs co-expressed higher levels of certain embryonic limb bud transcription factors, telomerase components and the p53 inhibitor Mdm4, and lower levels of myogenic differentiation markers compared to adult Pax7+ MuSCs, resulting in enhanced self-renewal and stress-response signatures. Functionally, conditional ablation and induction of Lin28a+ MuSCs in adult mice revealed that these cells are necessary and sufficient for efficient muscle regeneration. Together, our findings connect the embryonic factor Lin28a to adult stem cell self-renewal and juvenile regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 (1961).
Yin, H., Price, F. & Rudnicki, M. A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 93, 23–67 (2013).
von Maltzahn, J., Chang, N. C., Bentzinger, C. F. & Rudnicki, M. A. Wnt signaling in myogenesis. Trends Cell Biol. 22, 602–609 (2012).
Rossi, G. & Messina, G. Comparative myogenesis in teleosts and mammals. Cell Mol. Life Sci. 71, 3081–3099 (2014).
Kuang, S., Chargé, S. B., Seale, P., Huh, M. & Rudnicki, M. A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 172, 103–113 (2006).
Joe, A. W. B. et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153–163 (2010).
Mitchell, K. J. et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 12, 257–266 (2010).
Fry, C. S. et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 21, 76–80 (2015).
McCarthy, J. J. et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657–3666 (2011).
Kuang, S., Kuroda, K., Le Grand, F. & Rudnicki, M. A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010 (2007).
Walker, D. K. et al. PAX7+ satellite cells in young and older adults following resistance exercise. Muscle Nerve 46, 51–59 (2012).
Christensen, J. L. & Weissman, I. L. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA 98, 14541–14546 (2001).
Horvitz, H. R. & Sulston, J. E. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics 96, 435–454 (1980).
Sulston, J. E. & Horvitz, H. R. Abnormal cell lineages in mutants of the nematode Caenorhabditis elegans. Dev. Biol. 82, 41–55 (1981).
Ambros, V. & Horvitz, H. R. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226, 409–416 (1984).
Viswanathan, S. R., Daley, G. Q. & Gregory, R. I. Selective blockade of microRNA processing by Lin28. Science 320, 97–100 (2008).
Zhu, H. et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat. Genet. 42, 626–630 (2010).
Tsanov, K. M. et al. LIN28 phosphorylation by MAPK/ERK couples signalling to the post-transcriptional control of pluripotency. Nat. Cell Biol. 19, 60–67 (2017).
Yermalovich, A. V. et al. Lin28 and let-7 regulate the timing of cessation of murine nephrogenesis. Nat. Commun. 10, 168 (2019).
Osborne, J. K. et al. Lin28 paralogs regulate lung branching morphogenesis. Cell Rep. 36, 109408–109408 (2021).
Kerepesi, C., Zhang, B., Lee, S.-G., Trapp, A. & Gladyshev, V. N. Epigenetic clocks reveal a rejuvenation event during embryogenesis followed by aging. Sci. Adv. 7, eabg6082 (2021).
Shyh-Chang, N. et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 155, 778–792 (2013).
Polesskaya, A. et al. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 21, 1125–1138 (2007).
West, J. A. et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 460, 909–913 (2009).
Keefe, A. C. et al. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat. Commun. 6, 7087 (2015).
Seale, P. et al. Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786 (2000).
Lepper, C., Partridge, T. A. & Fan, C. M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646 (2011).
Der Vartanian, A. et al. PAX3 confers functional heterogeneity in skeletal muscle stem cell responses to environmental stress. Cell Stem Cell 24, 958–973.e9 (2019).
de Morree, A. et al. Alternative polyadenylation of Pax3 controls muscle stem cell fate and muscle function. Science 366, 734–738 (2019).
Schiaffino, S. & Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 (2011).
Liu, L. et al. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat. Protoc. 10, 1612–1624 (2015).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, 3156 (2013).
Horvath, S. & Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19, 371–384 (2018).
Motohashi, N. & Asakura, A. Muscle satellite cell heterogeneity and self-renewal. Front. Cell Dev. Biol. 2, 1 (2014).
Jaafar, R. et al. Phospholipase D regulates the size of skeletal muscle cells through the activation of mTOR signaling. Cell Commun. Signal. 11, 55 (2013).
Bober, E., Franz, T., Arnold, H. H., Gruss, P. & Tremblay, P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development 120, 603–612 (1994).
Yusuf, F. et al. Inhibitors of CXCR4 affect the migration and fate of CXCR4+ progenitors in the developing limb of chick embryos. Dev. Dyn. 235, 3007–3015 (2006).
Mercader, N. et al. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development 127, 3961–3970 (2000).
Capdevila, J., Tsukui, T., Esteban, C. R., Zappavigna, V. & Belmonte, J. C. I. Control of vertebrate limb outgrowth by the proximal factor Meis2 and distal antagonism of BMPs by Gremlin. Mol. Cell 4, 839–849 (1999).
Heanue, T. A. et al. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 13, 3231–3243 (1999).
Borsani, G. et al. EYA4, a novel vertebrate gene related to Drosophila eyes absent. Hum. Mol. Genet. 8, 11–23 (1999).
Grifone, R. et al. Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development 132, 2235–2249 (2005).
Jun-Hao, E. T., Gupta, R. R. & Shyh-Chang, N. Lin28 and let-7 in the metabolic physiology of aging. Trends Endocrinol. Metab. 27, 132–141 (2016).
Relaix, F., Rocancourt, D., Mansouri, A. & Buckingham, M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435, 948–953 (2005).
Tremblay, P. et al. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev. Biol. 203, 49–61 (1998).
Ridgeway, A. G. & Skerjanc, I. S. Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. J. Biol. Chem. 276, 19033–19039 (2001).
Grifone, R. et al. Eya1 and Eya2 proteins are required for hypaxial somitic myogenesis in the mouse embryo. Dev. Biol. 302, 602–616 (2007).
Yang, D. H. & Moss, E. G. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr. Patterns 3, 719–726 (2003).
Yokoyama, S. et al. Dynamic gene expression of Lin-28 during embryonic development in mouse and chicken. Gene Expr. Patterns 8, 155–160 (2008).
Buganim, Y. et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 150, 1209–1222 (2012).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Goodell, M. A., Nguyen, H. & Shroyer, N. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat. Rev. Mol. Cell Biol. 16, 299–309 (2015).
Muller-Sieburg, C. E., Sieburg, H. B., Bernitz, J. M. & Cattarossi, G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood 119, 3900–3907 (2012).
Boehm, M. & Slack, F. A developmental timing microRNA and its target regulate life span in C. elegans. Science 310, 1954–1957 (2005).
de Magalhães, J. P. Programmatic features of aging originating in development: aging mechanisms beyond molecular damage? FASEB J. 26, 4821–4826 (2012).
Darwin, C. The Variation of Animals and Plants Under Domestication, Vol. 1 (Cambridge University Press, 2010).
Poss, K. D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 11, 710–722 (2010).
Aalami, O. O. et al. Applications of a mouse model of calvarial healing: differences in regenerative abilities of juveniles and adults. Plast. Reconstr. Surg. 114, 713–720 (2004).
Detwiler, S. R. Restitution of the brachial region of the cord following unilateral excision in the embryo. J. Exp. Zool. 104, 53–68 (1947).
Holtzer, H. Reconstitution of the urodele spinal cord following unilateral ablation. Part I. Chronology of neuron regulation. J. Exp. Zool. 117, 523–557 (1951).
Davis, B. M., Duffy, M. T. & Simpson, S. B. Jr Bulbospinal and intraspinal connections in normal and regenerated salamander spinal cord. Exp. Neurol. 103, 41–51 (1989).
Wilson, A. A. et al. Lentiviral delivery of RNAi for in vivo lineage-specific modulation of gene expression in mouse lung macrophages. Mol. Ther. 21, 825–833 (2013).
Tasic, B. et al. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc. Natl. Acad. Sci. USA 108, 7902–7907 (2011).
Liu, T. M. et al. Ascorbate and iron are required for the specification and long-term self-renewal of human skeletal mesenchymal stromal cells. Stem Cell Reports 14, 210–225 (2020).
Acknowledgements
This work was supported by the Strategic Priority Research Program of the CAS (XDA16010109), the National Key R&D Program of China (2019YFA0801700), the National Natural Science Foundation of China (91957202), and the Key Research Program of the CAS (KJZD-SW-L04). N.S.-C. is also a Howard Hughes Medical Institute (HHMI) International Scholar.
Author information
Authors and Affiliations
Contributions
P.W. and X.L. designed and performed the experiments. P.W., X.L. and N.S.-C. wrote the manuscript. L. Guo helped with various experiments and mouse husbandry. J.-H.E.T., M.-W.J.C. and Y.-J.B.C. helped with HSKM and LTS cell experiments. L.L. helped with cryosection and immunofluorescence experiments. S.M. helped with Western blot experiments. W.C. helped with qRT-PCR experiments. W.M., N.A., Y.L. and L.J. helped with bioinformatics analysis. Z.Y., Y.C., K.L., L. Guang, Y.W., H.Z., R.R.W. and C.L. provided technical assistance. L.Z. and S.L. helped with mouse sampling. B.T.T., K.Y. and N.S.-C. designed and supervised the overall project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Liu, X., Yao, Z. et al. Lin28a maintains a subset of adult muscle stem cells in an embryonic-like state. Cell Res 33, 712–726 (2023). https://doi.org/10.1038/s41422-023-00818-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41422-023-00818-y