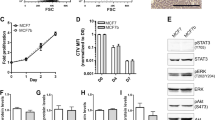
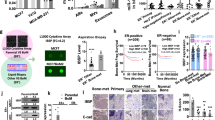
Abstract
Estrogen receptor (ER)-positive luminal breast cancer is a subtype with generally lower risk of metastasis to most distant organs. However, bone recurrence occurs preferentially in luminal breast cancer. The mechanisms of this subtype-specific organotropism remain elusive. Here we show that an ER-regulated secretory protein SCUBE2 contributes to bone tropism of luminal breast cancer. Single-cell RNA sequencing analysis reveals osteoblastic enrichment by SCUBE2 in early bone-metastatic niches. SCUBE2 facilitates release of tumor membrane-anchored SHH to activate Hedgehog signaling in mesenchymal stem cells, thus promoting osteoblast differentiation. Osteoblasts deposit collagens to suppress NK cells via the inhibitory LAIR1 signaling and promote tumor colonization. SCUBE2 expression and secretion are associated with osteoblast differentiation and bone metastasis in human tumors. Targeting Hedgehog signaling with Sonidegib and targeting SCUBE2 with a neutralizing antibody both effectively suppress bone metastasis in multiple metastasis models. Overall, our findings provide a mechanistic explanation for bone preference in luminal breast cancer metastasis and new approaches for metastasis treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The sequence data of osteoblasts were deposited in the National Omics Data Encyclopedia (NODE) with the primary accession number OEP002689. Original data of all figures were deposited in Mendeley Data (https://data.mendeley.com/v1/datasets/jkpn67jwsd/draft?preview=1). Other data of this study are available from the corresponding author upon reasonable request.
References
Zlotnik, A., Burkhardt, A. M. & Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 11, 597–606 (2011).
Roodman, G. D. Mechanisms of bone metastasis. N. Engl. J. Med. 350, 1655–1664 (2004).
Weilbaecher, K. N., Guise, T. A. & McCauley, L. K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer 11, 411–425 (2011).
Coleman, R. E. et al. Bone metastases. Nat. Rev. Dis. Primers 6, 83 (2020).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Sorlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 100, 8418–8423 (2003).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869–10874 (2001).
Rouzier, R. et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 11, 5678–5685 (2005).
Smid, M. et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 68, 3108–3114 (2008).
Savci-Heijink, C. D. et al. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res. Treat. 150, 547–557 (2015).
Kunikullaya, S. U., Poddar, J., Sharma, A. D. & Patel, S. Pattern of distant metastasis in molecular subtypes of carcinoma breast: An institutional study. Indian J. Cancer 54, 327–332 (2017).
Kennecke, H. et al. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 28, 3271–3277 (2010).
Farach-Carson, M. C., Lin, S. H., Nalty, T. & Satcher, R. L. Sex differences and bone metastases of breast, lung, and prostate cancers: do bone homing cancers favor feminized bone marrow? Front. Oncol. 7, 163 (2017).
Lee, S. J. et al. Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res. Treat. 43, 89–95 (2011).
Wang, J., Jarrett, J., Huang, C. C., Satcher, R. L. Jr. & Levenson, A. S. Identification of estrogen-responsive genes involved in breast cancer metastases to the bone. Clin. Exp. Metastasis 24, 411–422 (2007).
Wei, S., Li, Y., Siegal, G. P. & Hameed, O. Breast carcinomas with isolated bone metastases have different hormone receptor expression profiles than those with metastases to other sites or multiple organs. Ann. Diagn. Pathol. 15, 79–83 (2011).
Onishi, T., Hayashi, N., Theriault, R. L., Hortobagyi, G. N. & Ueno, N. T. Future directions of bone-targeted therapy for metastatic breast cancer. Nat. Rev. Clin. Oncol. 7, 641–651 (2010).
Zhang, X. H. et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16, 67–78 (2009).
Hayashi, N. et al. Bone metastasis-related signaling pathways in breast cancers stratified by estrogen receptor status. J. Cancer 8, 1045–1052 (2017).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Ell, B. & Kang, Y. SnapShot: bone metastasis. Cell 151, 690.e1 (2012).
Suva, L. J., Washam, C., Nicholas, R. W. & Griffin, R. J. Bone metastasis: mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 7, 208–218 (2011).
Zhuang, X. et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol. 19, 1274–1285 (2017).
Coleman, R. E. Zoledronic acid ameliorates the effects of endocrine therapy on bone health in women with early-stage breast cancer. Nat. Clin. Pract. Endocrinol. Metab. 5, 72–73 (2009).
Mackiewicz-Wysocka, M., Pankowska, M. & Wysocki, P. J. Progress in the treatment of bone metastases in cancer patients. Expert Opin. Investig. Drugs 21, 785–795 (2012).
Haider, M. T., Holen, I., Dear, T. N., Hunter, K. & Brown, H. K. Modifying the osteoblastic niche with zoledronic acid in vivo-Potential implications for breast cancer bone metastasis. Bone 66, 240–250 (2014).
Devignes, C. S. et al. HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc. Natl. Acad. Sci. USA 115, E992–E1001 (2018).
Bussard, K. M., Venzon, D. J. & Mastro, A. M. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. J. Cell. Biochem. 111, 1138–1148 (2010).
Wang, H. et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27, 193–210 (2015).
Wang, H. et al. The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell 34, 823–839.e7 (2018).
Zheng, H. et al. Therapeutic antibody targeting tumor- and osteoblastic niche-derived jagged1 sensitizes bone metastasis to chemotherapy. Cancer Cell 32, 731–747.e6 (2017).
Sethi, N., Dai, X., Winter, C. G. & Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19, 192–205 (2011).
Kolb, A. D., Shupp, A. B., Mukhopadhyay, D., Marini, F. C. & Bussard, K. M. Osteoblasts are "educated" by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment. Breast Cancer Res. 21, 31 (2019).
Grimmond, S. et al. Cloning, mapping, and expression analysis of a gene encoding a novel mammalian EGF-related protein (SCUBE1). Genomics 70, 74–81 (2000).
Kawakami, A. et al. The zebrafish-secreted matrix protein you/scube2 is implicated in long-range regulation of hedgehog signaling. Curr. Biol. 15, 480–488 (2005).
Johnson, J. L. et al. Scube activity is necessary for Hedgehog signal transduction in vivo. Dev. Biol. 368, 193–202 (2012).
Woods, I. G. & Talbot, W. S. The you gene encodes an EGF-CUB protein essential for Hedgehog signaling in zebrafish. PLoS Biol. 3, e66 (2005).
Creanga, A. et al. Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form. Genes Dev. 26, 1312–1325 (2012).
Parchure, A., Vyas, N. & Mayor, S. Wnt and Hedgehog: secretion of lipid-modified morphogens. Trends Cell Biol. 28, 157–170 (2018).
Jakobs, P. et al. Scube2 enhances proteolytic Shh processing from the surface of Shh-producing cells. J. Cell Sci. 127, 1726–1737 (2014).
Wierbowski, B. M. et al. Hedgehog pathway activation requires coreceptor-catalyzed, lipid-dependent relay of the Sonic Hedgehog ligand. Dev. Cell 55, 450–467. e8 (2020).
Tukachinsky, H., Kuzmickas, R. P., Jao, C. Y., Liu, J. & Salic, A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2, 308–320 (2012).
Tsai, M. T. et al. Isolation and characterization of a secreted, cell-surface glycoprotein SCUBE2 from humans. Biochem. J. 422, 119–128 (2009).
Petrov, K., Wierbowski, B. M., Liu, J. & Salic, A. Distinct cation gradients power cholesterol transport at different key points in the Hedgehog signaling pathway. Dev. Cell 55, 314–327.e317 (2020).
Smid, M. et al. Genes associated with breast cancer metastatic to bone. J. Clin. Oncol. 24, 2261–2267 (2006).
Minn, A. J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Wang, Y. et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365, 671–679 (2005).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Minn, A. J. et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc. Natl. Acad. Sci. USA 104, 6740–6745 (2007).
Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Richardson, A. L. et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell 9, 121–132 (2006).
Lelekakis, M. et al. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis 17, 163–170 (1999).
Paul, F. et al. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell 163, 1663–1677 (2015).
Tusi, B. K. et al. Population snapshots predict early haematopoietic and erythroid hierarchies. Nature 555, 54–60 (2018).
Nikolic, T., Dingjan, G. M., Leenen, P. J. & Hendriks, R. W. A subfraction of B220(+) cells in murine bone marrow and spleen does not belong to the B cell lineage but has dendritic cell characteristics. Eur. J. Immunol. 32, 686–692 (2002).
Giladi, A. et al. Single-cell characterization of haematopoietic progenitors and their trajectories in homeostasis and perturbed haematopoiesis. Nat. Cell Biol. 20, 836–846 (2018).
Wiraja, C., Yeo, D. C., Chong, M. S. & Xu, C. Nanosensors for continuous and noninvasive monitoring of mesenchymal stem cell osteogenic differentiation. Small 12, 1342–1350 (2016).
Corada, M. et al. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 97, 1679–1684 (2001).
Lin, Y. C. et al. Endothelial SCUBE2 interacts With VEGFR2 and regulates VEGF-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 37, 144–155 (2017).
Huang, W., Yang, S., Shao, J. & Li, Y. P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 12, 3068–3092 (2007).
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013).
Kim, W. K., Meliton, V., Bourquard, N., Hahn, T. J. & Parhami, F. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J. Cell Biochem. 111, 1199–1209 (2010).
Li, L. et al. Hedgehog signaling is involved in the BMP9-induced osteogenic differentiation of mesenchymal stem cells. Int. J. Mol. Med. 35, 1641–1650 (2015).
Zhao, Q. et al. Identification of genes expressed with temporal-spatial restriction to developing cerebellar neuron precursors by a functional genomic approach. Proc. Natl. Acad. Sci. USA 99, 5704–5709 (2002).
Yauch, R. L. et al. A paracrine requirement for hedgehog signalling in cancer. Nature 455, 406–410 (2008).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell. Biol. 15, 786–801 (2014).
Kaur, A. et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9, 64–81 (2019).
Egeblad, M., Rasch, M. G. & Weaver, V. M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell. Biol. 22, 697–706 (2010).
Meyaard, L. et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 7, 283–290 (1997).
Meyaard, L. The inhibitory collagen receptor LAIR-1 (CD305). J. Leukoc. Biol. 83, 799–803 (2008).
Lebbink, R. J. et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 203, 1419–1425 (2006).
Peng, D. H. et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat. Commun. 11, 4520 (2020).
Rygiel, T. P., Stolte, E. H., de Ruiter, T., van de Weijer, M. L. & Meyaard, L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol. Immunol. 49, 402–406 (2011).
Kuczek, D. E. et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 7, 68 (2019).
Boraschi-Diaz, I. et al. Collagen type I degradation fragments act through the collagen receptor LAIR-1 to provide a negative feedback for osteoclast formation. Bone 117, 23–30 (2018).
Theocharis, A. D., Skandalis, S. S., Gialeli, C. & Karamanos, N. K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 97, 4–27 (2016).
Lebbink, R. J. et al. Mouse leukocyte-associated Ig-like receptor-1 (mLAIR-1) functions as an inhibitory collagen-binding receptor on immune cells. Int. Immunol. 19, 1011–1019 (2007).
Jansen, C. A. et al. Regulated expression of the inhibitory receptor LAIR-1 on human peripheral T cells during T cell activation and differentiation. Eur. J. Immunol. 37, 914–924 (2007).
Rubin, L. L. & de Sauvage, F. J. Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 5, 1026–1033 (2006).
Takebe, N. et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 12, 445–464 (2015).
Sekulic, A. & Hoff, Von D. Hedgehog pathway inhibition. Cell 164, 831 (2016).
Cortes, J. E., Gutzmer, R., Kieran, M. W. & Solomon, J. A. Hedgehog signaling inhibitors in solid and hematological cancers. Cancer Treat. Rev. 76, 41–50 (2019).
Haley, H. R. et al. Enhanced bone metastases in skeletally immature mice. Tomography 4, 84–93 (2018).
Eckhardt, B. L. et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol. Cancer Res. 3, 1–13 (2005).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Olechnowicz, S. W. & Edwards, C. M. Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res. 74, 1625–1631 (2014).
Kim, R. S. et al. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS One 7, e35569 (2012).
Chen, J. H. et al. Upregulated SCUBE2 expression in breast cancer stem cells enhances triple negative breast cancer aggression through modulation of notch signaling and epithelial-to-mesenchymal transition. Exp. Cell Res. 370, 444–453 (2018).
Lin, Y. C., Lee, Y. C., Li, L. H., Cheng, C. J. & Yang, R. B. Tumor suppressor SCUBE2 inhibits breast-cancer cell migration and invasion through the reversal of epithelial-mesenchymal transition. J. Cell Sci. 127, 85–100 (2014).
Cheng, C. J. et al. SCUBE2 suppresses breast tumor cell proliferation and confers a favorable prognosis in invasive breast cancer. Cancer Res. 69, 3634–3641 (2009).
Cong, M. et al. MTSS1 suppresses mammary tumor-initiating cells by enhancing RBCK1-mediated p65 ubiquitination. Nat. Cancer 1, 222–234 (2020).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018).
Ambrosi, T. H. et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 597, 256–262 (2021).
Baryawno, N. et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell 177, 1915–1932.e16 (2019).
Gupta, S. & Maecker, H. Intracellular cytokine staining (ICS) on human lymphocytes or peripheral blood mononuclear cells (PBMCs). Bio. Protoc. 5, e1442 (2015).
Zhang, P. et al. SH3RF3 promotes breast cancer stem-like properties via JNK activation and PTX3 upregulation. Nat. Commun. 11, 2487 (2020).
Fleming, J. M., Miller, T. C., Meyer, M. J., Ginsburg, E. & Vonderhaar, B. K. Local regulation of human breast xenograft models. J. Cell. Physiol. 224, 795–806 (2010).
Acknowledgements
We would like to thank Kai Wang, Shuyang Yan, Xiang Miao, Yiting Yuan, Jun Li, Zhonghui Weng, Yujia Zhai, Yifan Bu from Institutional Center for Shared Technologies and Facilities of SINH, CAS for technical assistance. The study was funded by the National Natural Science Foundation of China (82230096, 82003090), the National Key R&D Program of China (2020YFA0112300), the Science and Technology Commission of Shanghai Municipality (19JC1416100) and the Youth Innovation Promotion Association of Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
G.H. supervised this work. Q.W. and G.H. drafted the manuscript. Q.W., P.T., D.H., Z.J., Y.H., W.L., X.L., Y.W., P.Z. and Y.L. performed the experiments. W.Z., P.S., J.Q., Y.-Z.J., Z.-M.S. and Q.Y. contributed to clinical sample collection and analyses. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Q., Tian, P., He, D. et al. SCUBE2 mediates bone metastasis of luminal breast cancer by modulating immune-suppressive osteoblastic niches. Cell Res 33, 464–478 (2023). https://doi.org/10.1038/s41422-023-00810-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41422-023-00810-6
This article is cited by
-
The Role of Breast Cancer Cells in Bone Metastasis: Suitable Seeds for Nourishing Soil
Current Osteoporosis Reports (2024)
-
Prognostic analysis of percutaneous vertebroplasty (PVP) combined with 125I implantation on lumbosacral vertebral osteoblastic metastases
World Journal of Surgical Oncology (2023)
-
New insights into the correlations between circulating tumor cells and target organ metastasis
Signal Transduction and Targeted Therapy (2023)