Abstract
Human NAD-dependent isocitrate dehydrogenase or HsIDH3 catalyzes the decarboxylation of isocitrate into α-ketoglutarate in the TCA cycle. HsIDH3 exists and functions as a heterooctamer composed of the αβ and αγ heterodimers, and is regulated allosterically and/or competitively by numerous metabolites including CIT, ADP, ATP, and NADH. In this work, we report the crystal structure of HsIDH3 containing a β mutant in apo form. In the HsIDH3 structure, the αβ and αγ heterodimers form the α2βγ heterotetramer via their clasp domains, and two α2βγ heterotetramers form the (α2βγ)2 heterooctamer through insertion of the N-terminus of the γ subunit of one heterotetramer into the back cleft of the β subunit of the other heterotetramer. The functional roles of the key residues at the allosteric site, the pseudo allosteric site, the heterodimer and heterodimer–heterodimer interfaces, and the N-terminal of the γ subunit are validated by mutagenesis and kinetic studies. Our structural and biochemical data together demonstrate that the allosteric site plays an important role but the pseudo allosteric site plays no role in the allosteric activation of the enzyme; the activation signal from the allosteric site is transmitted to the active sites of both αβ and αγ heterodimers via the clasp domains; and the N-terminal of the γ subunit plays a critical role in the formation of the heterooctamer to ensure the optimal activity of the enzyme. These findings reveal the molecular mechanism of the assembly and allosteric regulation of HsIDH3.
Similar content being viewed by others
Introduction
In all aerobic organisms, the cells use the tricarboxylic acid (TCA) cycle (also called citric acid cycle or Krebs cycle) to generate ATP through oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. In addition, the TCA cycle also provides intermediates for de novo synthesis of proteins, lipids and nucleic acids1. Among a series of biochemical reactions in the TCA cycle, isocitrate dehydrogenases (IDHs) catalyze oxidative decarboxylation of isocitrate (ICT) into α-ketoglutarate (α-KG) using NAD or NADP as coenzyme. Most prokaryotes contain only NADP-dependent IDHs (NADP-IDHs) in the cytosol to exert the catalytic function. Eukaryotes contain both NADP-IDHs and NAD-dependent IDHs (NAD-IDHs). In human and other mammalian cells, there are two NADP-IDHs, which are located to the cytosol and the mitochondria, and one NAD-IDH which is located to the mitochondria. Human (Homo sapien) NADP-IDHs and NAD-IDH are also called HsIDH1, HsIDH2, and HsIDH3, respectively. It is well established that HsIDH3 exerts the catalytic function in the TCA cycle2, whereas HsIDH1 and HsIDH2 play important roles in cellular defense against oxidative damage3, removal of reactive oxygen species4, and synthesis of fat and cholesterol5. Aberrant functions of all three enzymes have been implicated in the pathogenesis of numerous metabolic diseases6,7,8 and malignant tumors9,10,11,12.
Both prokaryotic13 and eukaryotic14,15 NADP-IDHs exist and function as homodimers in which both subunits have catalytic activity. These enzymes share a conserved catalytic mechanism, but have different regulatory mechanisms. The activity of Escherichia coli NADP-IDH is regulated through reversible phosphorylation and dephosphorylation of a strictly conserved Ser at the active site by the dual functional kinase/phosphatase AceK, and other bacterial NADP-IDHs might share a similar regulatory mechanism16,17. The activity of HsIDH1 is regulated through substrate-binding induced conformational change of a key structure element at the active site, and other mammalian NADP-IDHs might utilize a similar regulatory mechanism14,18.
Compared to NADP-IDHs, NAD-IDHs are composed of different types of subunits with distinct functions and employ more sophisticated regulatory mechanisms. Saccharomyces cerevisiae NAD-IDH is composed of a regulatory subunit ScIDH1 and a catalytic subunit ScIDH2, which form the ScIDH1/ScIDH2 heterodimer as the basic functional unit19, and the heterodimer is assembled into a heterotetramer and further into a heterooctamer20,21. ScIDH2 contains the active site and ScIDH1 contains the allosteric site, and the binding of activators citrate (CIT) and AMP to the allosteric site causes conformational changes of the active site through the heterodimer interface, leading to activation of the enzyme20. The composition and regulation of mammalian NAD-IDHs are more complex than those of yeast NAD-IDH. Mammalian NAD-IDH is composed of three types of subunits in the ratio of 2αː1βː1γ 22,23. The α, β, and γ subunits have a molecular mass of about 37, 39, and 39 kDa, respectively; and the α and β subunits share about 40% sequence identity, the α and γ subunits about 42% sequence identity, and the β and γ subunits about 52% sequence identity. The α and β subunits form a heterodimer (αβ) and the α and γ subunits form another heterodimer (αγ), and the two heterodimers are assembled into the α2βγ heterotetramer and further into the (α2βγ)2 heterooctamer24. Early biochemical studies of mammalian NAD-IDHs showed that the α subunit is the catalytic subunit25, and the β and γ subunits are the regulatory subunits26; and the activity is positively regulated by CIT27 and ADP28, but negatively regulated by ATP and NADH29. The functional roles of several strictly conserved residues of the α, β, and γ subunits of HsIDH3 in the bindings of metal ion, ICT, NAD, and ADP and the catalytic reaction were also examined by biochemical studies, and the results showed that the α subunit is critical for the catalytic activity, and the β and γ subunits play important roles in the allosteric regulation30,31,32,33,34.
Our biochemical and structural studies of HsIDH3 confirmed some results from the previous studies but also revealed some new findings. We found that the α subunits of both αβ and αγ heterodimers have catalytic activity; however, only the γ subunit plays a regulatory role via an allosteric regulatory mechanism, while the β subunit plays no regulatory role but is required for the optimal function of HsIDH335. The αγ heterodimer are positively regulated by CIT and ADP36, and negatively regulated by NADH37. In addition, these enzymes can be activated by low concentrations of ATP but inhibited by high concentrations of ATP38. In contrast, the αβ heterodimer cannot be activated by CIT and ADP and is inhibited by both NADH and ATP39. Our findings revealed the underlying molecular mechanisms of the αγ and αβ heterodimers. Nevertheless, so far the structure, assembly and regulatory mechanism of HsIDH3 remain unknown. Thus, how the αβ and αγ heterodimers are assembled into the α2βγ heterotetramer and further into the (α2βγ)2 heterooctamer is unclear. How the allosteric site in the γ subunit regulates both α subunits in the α2βγ heterotetramer is also unclear. Whether the regulatory mechanisms of the αβ and αγ heterodimers are applicable to the heterooctamer is elusive.
In this work, we determined the crystal structure of HsIDH3 containing a β mutant in apo form. In the apo HsIDH3 structure, the αβ and αγ heterodimers assemble the α2βγ heterotetramer via their clasp domains, and two α2βγ heterotetramers assemble the (α2βγ)2 heterooctamer through insertion of the N-terminal of the γ subunit of one heterotetramer into the back cleft of the β subunit of the other heterotetramer. We also performed mutagenesis and kinetic studies to validate the functional roles of key residues at the allosteric site, the pseudo allosteric site, the heterodimer interface, and the heterodimer–heterodimer interface, as well as the N-terminal of the γ subunit. Our structural and biochemical data together reveal the molecular mechanism for the assembly and allosteric regulation of HsIDH3.
Results
Preparation and biochemical analysis of HsIDH3
Crystallization of wild-type HsIDH3 yielded crystals which diffracted X-rays to low resolution (about 10 Å), prohibiting us from determining the crystal structure. Our previous biochemical and structural studies showed that substitution of the C-terminal of the β subunit (residues 341–349) with that of the α subunit (residues 330–338) produced a stable αβ mutant which exhibits similar enzymatic properties as wild-type αβ heterodimer, and this αβ mutant yielded high quality crystals which allowed us to solve the structure of the αβ heterodimer39. Thus, we prepared a mutant HsIDH3 containing this β mutant, which led to successful structure determination of HsIDH3.
Like wild-type HsIDH3, the β-mutant HsIDH3 exists as a stable heterooctamer in solution with high purity and homogeneity as shown by SEC, SDS-PAGE (Supplementary Fig. S1), and SEC-MALS analyses (Supplementary Fig. S2c, d and Table S1). The β-mutant HsIDH3 exhibits almost identical enzymatic properties as wild-type HsIDH3 (Supplementary Fig. S3a, b and Table S2). These results indicate that the substitution of the C-terminal of the β subunit has no notable effects on the biochemical and enzymatic properties of HsIDH3.
Crystal structure of HsIDH3 in apo form
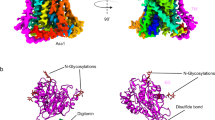
The crystal structure of the β-mutant HsIDH3 was solved at 3.47 Å resolution with each asymmetric unit containing one α2βγ heterotetramer (Table 1). The four polypeptide chains of the heterotetramer are largely well-defined with good electron density except for a few N-terminal and/or C-terminal residues, and the α, β, and γ subunits can be distinguished unambiguously based on the differences of numerous residues with large side chains (Supplementary Fig. S4). There are no ligands bound at the active sites, the allosteric site, and the pseudo allosteric site; thus, this structure represents the apo HsIDH3. In the apo β-mutant HsIDH3 structure, the αβ and αγ heterodimers assume very similar overall structures as those in the isolated forms36,39 (Fig. 1). All of the α, β, and γ subunits consist of 10 α-helices and 12 β-strands, which fold into a large domain, a small domain, and a clasp domain. Both of the αβ and αγ heterodimers have a pseudo two-fold symmetry along the heterodimer interface. The heterodimer interface is mediated by the α6 and α7 helices of the small domains which form a four-helix bundle in a parallel manner, and the β6 and β7 strands of the clasp domains (clasp β-strands), which form a four-stranded β-sheet (clasp β-sheet) in an antiparallel manner. The heterodimer interface buries about 2180 Å2 and 2094 Å2 solvent accessible surface or 13.7% and 13.5% of the surface area of each subunit in the αβ and αγ heterodimers, respectively, indicating that the heterodimer interface is very tight in both heterodimers.
a Surface and b cartoon presentations of the apo β-mutant HsIDH3 in two different orientations. Left: view along the crystallographic 2-fold axis of the heterooctamer of HsIDH3. Right: view in perpendicular to the crystallographic 2-fold axis of the heterooctamer of HsIDH3. The color coding of the α, β, and γ subunits is shown above. The N-terminal regions of the γ subunits and the C-terminal substituted regions of the β subunits are colored in blue and red, respectively. The clasp domains of the αβ and αγ heterodimers are indicated with dashed ovals. The active sites, the allosteric sites, and the pseudo allosteric sites are indicated with yellow, red, and orange spheres, respectively. Residues and structure elements of the α and β subunits of the αβ heterodimer and the α and γ subunits of the αγ heterodimer are superscripted by “A1” and “B”, and “A2” and “G”, respectively.
Nevertheless, the αβ and αγ heterodimers in the apo β-mutant HsIDH3 structure also exhibit some notable conformational differences from those in the isolated forms36,39. In particular, the αβ heterodimer assumes an open overall conformation similar to that of the isolated αγ heterodimer rather than the compact conformation of the isolated αβ heterodimer, rendering it suitable for allosteric activation and catalytic reaction (see discussion later). In addition, the N-terminal (residues 1–14) of the γ subunit is disordered in all of our previously determined αγ structures regardless of the presence or absence of ligands36,37,38; however, a large portion of the N-terminal region (residues 5–14) of the αγ heterodimer is well-defined in the heterooctamer, which plays an important role in the formation and function of the heterooctamer (see discussion later). It is also noteworthy that the C-terminal of the β subunit is located on the structure surface and involved in the crystal packing, but is not involved in the assembly of the heterodimer, the heterotetramer, and the heterooctamer (Fig. 1). This explains why the crystals of the β-mutant HsIDH3 diffracted X-rays better than those of wild-type HsIDH3, and the β-mutant HsIDH3 has comparable biochemical and enzymatic properties as wild-type HsIDH3.
The heterodimer–heterodimer interface in the α2βγ heterotetramer
In the structure of the β-mutant HsIDH3, the α2βγ heterotetramer is assembled by the αβ and αγ heterodimers via their clasp domains (Figs. 1 and 2a). There is a pseudo two-fold symmetry along the heterodimer–heterodimer interface, which is about 25° off the coplane axes of the αβ and αγ heterodimers. In other words, the coplane axes of the αβ and αγ heterodimers make a 50° angle. Thus, the heterotetramer has a distorted tetrahedron architecture with the two α subunits occupying two vertices on the same side and the β and γ subunits two vertices on the other side (Fig. 2a). The heterodimer–heterodimer interface buries about 804 Å2 solvent accessible surface or 3.0% of the surface area of each heterodimer. At the interface, the clasp β-sheets of the two heterodimers interact with each other to form a β-barrel in a reciprocal manner such that the clasp β-strands of the β subunit stack antiparallelly onto those of the α subunit of the αγ heterodimer, and the clasp β-strands of the γ subunit stack antiparallelly onto those of the α subunit of the αβ heterodimer (Fig. 2a, b). The interface consists of 22 hydrophobic residues and two Ser residues from the four clasp domains, which form extensive hydrophobic interactions (Fig. 2b). In addition, there are eight hydrophilic residues which form two layers of hydrophilic interactions to separate the hydrophobic interactions (Fig. 2b, c). Specifically, the side chains of His131A1 and Gln139A1 of the α subunit in the αβ heterodimer and His131A2 and Gln139A2 of the α subunit in the αγ heterodimer form one layer of hydrogen bonds, and the side chains of Glu150B and His142B of the β subunit and Glu148G and His140G of the γ subunit form another layer of hydrogen bonds (residues and structure elements of the α and β subunits of the αβ heterodimer and the α and γ subunits of the αγ heterodimer are superscripted by “A1” and “B”, and A2” and “G”, respectively). Sequence alignments showed that these residues are strictly or highly conserved in other mammalian NAD-IDHs and yeast NAD-IDH35,39 (Supplementary Fig. S5), suggesting that other eukaryotic NAD-IDHs might form the heterotetramers in a similar manner.
a The α2βγ heterotetramer is assembled by the αβ and αγ heterodimers via their clasp domains. The color coding of the α, β, and γ subunits is the same as in Fig. 1. The pseudo 2-fold axis along the heterodimer–heterodimer interface, and the coplane axes of the αβ and αγ heterodimers are indicated. b Structure of the heterodimer–heterodimer interface. Middle panel: interactions at the interface consist of largely hydrophobic residues (marked by blue ovals) and a few hydrophilic residues (marked by black rectangles). Upper panel: interactions between the α and β subunits at the interface. Lower panel: interactions between the α and γ subunits at the interface. c Hydrogen-bonding interactions between the β and γ subunits (left panel) and between the two α subunits (right panel). d A surface diagram showing that the N-terminal region of the γ subunit (in cyan ribbon) of one heterotetramer lies in a shallow cleft (the “back cleft”) formed by the small and large domains of the β subunit (in green and dark green surface, respectively) of the other heterotetramer. e Interactions between the γ subunit of one heterotetramer (in cyan) and the β subunit of the other heterotetramer (in green). The hydrogen-bonding interactions are indicated with dashed lines.
The heterotetramer–heterotetramer interface in the (α2βγ)2 heterooctamer
In the structure of the β-mutant HsIDH3, the (α2βγ)2 heterooctamer is assembled by two α2βγ heterotetramers related by a crystallographic two-fold symmetry via the β and γ subunits (Fig. 1). Thus, the heterooctamer has a distorted tetrahedron architecture without a pseudo 222 symmetry. Specifically, the two β and two γ subunits are arranged alternately to form the inner core, and the four α subunits are positioned on the periphery. The heterotetramer–heterotetramer interface buries about 2248 Å2 solvent accessible surface or 4.3% of the surface area of the heterotetramer. At the interface, the N-terminal of the γ subunit of one heterotetramer intrudes into a shallow cleft between the small and large domains of the β subunit on the back of the pseudo allosteric site (“back cleft”) of the other heterotetramer (Fig. 2d). In particular, residues 10–14 (AKYGG) of the γ subunit make several hydrogen-bonding interactions with residues of the back cleft of the β subunit, which form a major part of the heterotetramer–heterotetramer interface (Fig. 2e). Specifically, the main chain of Pro8G forms a hydrogen bond with the side chain of Lys165B; the side chain of Tyr12G forms a hydrogen bond with the side chain of Asp169B and a π–π stacking interaction with the side chain of Phe166B; the main chain of Gly13G form a hydrogen bond each with the main chain of Ala264B and the side chain of His301B; the main chain of Gly14G forms a hydrogen bond with the main chain of Glu265B. In addition to the N-terminal, the α2 helix of the γ subunit also makes interactions with the α4 and α5 helices of the β subunit, which form a minor part of the heterotetramer–heterotetramer interface. In this region, the main chain and side chain of Arg70G form a hydrogen bond each with the side chain of Lys165B and the main chain of Leu205B, respectively. Sequence alignments showed that although the N-terminal of the γ subunit is different from that of the α and β subunits, residues 10–14 (AKYGG) are strictly or highly conserved in the regulatory subunits of other mammalian and yeast NAD-IDHs35,39 (Supplementary Fig. S5), suggesting that the N-terminal of the regulatory subunit in other eukaryotic NAD-IDHs might play a similar role in the assembly of the heterooctamer.
The apo HsIDH3 structure assumes an inactive conformation
Our previous structural studies of the isolated αγ and αβ heterodimers show that the αγ heterodimer contains an allosteric site in the γ subunit to bind the activators CIT and ADP, and the binding of CIT and ADP induces conformational changes at the allosteric site which are transmitted to the active site via the heterodimer interface36. In the αMgγ structure (PDB code: 5GRH), the side chain of Tyr135G at the allosteric site assumes a conformation unsuitable for the CIT binding, the N-terminal region of the α7 helix at the heterodimer interface in both the α and γ subunits adopts a loop conformation, and the side chain of Tyr126A2 at the active site assumes a conformation unsuitable for the substrate ICT binding; and thus, the αMgγ structure is considered to assume an inactive conformation36. On the other hand, in the αMgγMg+CIT+ADP structure (PDB code: 5GRF), the side chain of Tyr135G at the allosteric site assumes a conformation to bind the CIT, the N-terminal region of the α7 helix in both the α and γ subunits adopts an α-helical conformation, and the side chain of Tyr126A2 at the active site assumes a conformation suitable for the ICT binding; and thus, the αMgγMg+CIT+ADP structure is considered to assume an active conformation36. In contrast, the αβ heterodimer contains a pseudo allosteric site in the β subunit, which is structurally different from the allosteric site and hence is unable to bind the activators39. In the αCaβ structure (PDB code: 6KDE), the N-terminal region of the α7 helix in both the α and β subunits assumes an α-helical conformation, and the key residues Tyr137B at the pseudo allosteric site (equivalent to Tyr135G at the allosteric site) and Tyr126A1 at the active site also assume the active conformations similar to those in the αMgγMg+CIT+ADP heterodimer; and therefore, the αCaβ structure is considered to assume an active conformation39. However, in the αCaβ structure, the αβ heterodimer assumes a more compact overall structure than the αγ heterodimer due to the rotation of the β subunit towards the α subunit, which leads to conformational changes of the heterodimer interface and consequently yields a distorted active site that cannot bind the metal ion appropriately in a catalysis relevant manner39. These results revealed the underlying molecular mechanisms of the isolated αγ and αβ heterodimers.
Structural comparison shows that in the apo β-mutant HsIDH3 structure, the αγ heterodimer adopts an overall conformation similar to that in the αMgγ structure with the inactive conformation rather than that in the αMgγMg+CIT+ADP structure with the active conformation36. In particular, the key residues at the active site (Tyr126A2) and the allosteric site (Tyr135G) assume inactive conformations, and the N-terminal regions of both α7A2 and α7 G helices at the heterodimer interface assume inactive loop conformations similar to those in the αMgγ structure (Fig. 3a). Intriguingly, the αβ heterodimer exhibits some conformational differences from that in the αCaβ structure39. Structural analysis reveals that the insertion of the N-terminal of the γ subunit into the back cleft of the β subunit pushes the large domain of the β subunit to rotate away from the α subunit (the structure elements moving away from the α subunit by about 1.5–3 Å) (Fig. 3b). Consequently, the αβ heterodimer assumes an open overall conformation similar to that of the αMgγ structure rather than the compact conformation of the αCaβ structure. In addition, the key residues at the active site (Tyr126A1) and the pseudo allosteric site (Tyr137B) also assume inactive conformations similar to those in the αMgγ structure (Fig. 3c). The N-terminal region of helix α7A1 of the α subunit at the heterodimer interface assumes a loop conformation, but the N-terminal region of helix α7B of the β subunit assumes an α-helical conformation. At the pseudo allosteric site, although the β3B–α3B loop is disordered similar to that in the αCaβ structure, the β12B–α8B loop exhibits some conformational differences from that in the αCaβ structure and in particular the N-terminal region of the β12B–α8B loop maintains interactions with the α6A1 and α7B helices at the heterodimer interface and still occupies the ADP-binding site, prohibiting the ADP binding (Fig. 3d). These results show that the formation of the heterooctamer renders the αβ heterodimer to adopt an overall conformation similar to that of the αγ heterodimer; however, the pseudo allosteric site remains incapable of binding the activators and thus the β subunit still has no regulatory function, explaining why the αβ heterodimer in the heterooctamer of HsIDH3 can be activated and has normal catalytic activity but cannot bind the activators. Taken together, our structural data indicate that the apo β-mutant HsIDH3 structure assumes an inactive conformation as both of the αβ heterodimer and the αγ heterodimer adopt inactive conformations similar to that in the αMgγ structure.
a Comparison of the αγ heterodimer in the heterooctamer of HsIDH3 and in the isolated forms at the heterodimer interface. The color coding of the subunits and structures is shown above. The key residues at the active site (Tyr126A2), the allosteric site (Arg97G, Tyr135G, and Arg272G), and the heterodimer interface (Lys151G and Lys142A2) assume similar conformations as those in the inactive αMgγ structure (PDB code: 5GRH) rather than those in the active αMgγMg+CIT+ADP structure (PDB code: 5GRF). b Comparison of the overall conformation of the αβ heterodimer in the heterooctamer of HsIDH3 with that of the isolated αCaβ heterodimer (PDB code: 6KDE) (in gray, left panel) and αMgγ heterodimer (in yellow, right panel). The αβ heterodimer assumes an open overall conformation similar to that of the isolated αMgγ heterodimer rather than the compact conformation of the isolated αCaβ heterodimer. For clarity, only the α helices and β strands are shown, and the loops are omitted except the β12-β8 loops of the β and γ subunits. The N-terminal of the γ subunit from another heterotetramer is also shown. c Comparison of the αβ heterodimer in the heterooctamer of HsIDH3 with the isolated αMgγ heterodimer and αMgγMg+CIT+ADP heterodimer. The key residues at the active site (Tyr126A1), the pseudo allosteric site (Arg99B, Tyr137B, and Arg274B), and the heterodimer interface (Lys153B and Lys142A1) assume similar conformations as those in the inactive αMgγ structure rather than those in the active αMgγMg+CIT+ADP structure. d The β12B-β8B loop of the β subunit in the heterooctamer of HsIDH3 exhibits some conformational differences from that in the isolated αCaβ heterodimer but still occupies the ADP-binding site in the αMgγMg+CIT+ADP structure. The hydrogen-bonding interactions of the β12B-β8B loop with the α7B and α6A1 helices are indicated with dashed lines.
Functional roles of key residues in the assembly and allosteric regulation of HsIDH3
Our previous biochemical and structural studies of the αβ and αγ heterodimers showed that residues Arg97G, Tyr135G, and Arg272G at the allosteric site, and residues Lys151G and Lys142A2 at the heterodimer interface play important roles in the allosteric regulation36, whereas residues Arg99B, Tyr137B, and Arg274B at the pseudo allosteric site play no notable role in the allosteric regulation35,39. Analysis of the apo β-mutant HsIDH3 structure also shows that residues His131A1, Gln139A1, His131A2, Gln139A2, His142B, Glu150B, His140G, and Glu148G of the clasp domains play an important role in the assembly of the α2βγ heterotetramer (Fig. 2c). To investigate the functional roles of these residues in the allosteric regulation of HsIDH3, we prepared a series of HsIDH3 mutants containing point mutations of the key residues at the allosteric site (γR97A, γY135A and γR272A) (Fig. 3a), the pseudo allosteric site (βR99A, βY137A and βR274A) (Fig. 3c), the heterodimer interfaces (α1K142A, α2K142A, α1K142Aα2K142A, βK153A, and γK151A) (Fig. 3a,c), and the heterodimer–heterodimer interface (α1Q139A, α2Q139A, α1Q139Aα2Q139A, βE150A, and γE148A) (Fig. 2c), and measured their kinetic parameters in the absence or presence of CIT and ADP to examine the effects of the mutations on the activity and allosteric activation of HsIDH3. Most of the HsIDH3 mutants could be expressed and purified well, and exist as heterooctamer in solution with high purity and homogeneity as shown by SEC and SDS-PAGE analyses (Supplementary Fig. S1). The mutant αβ and αγ heterodimers containing mutations α1H131A, α2H131A, βH142A, and γH140A could not be expressed and thus the HsIDH3 mutants containing these mutations could not be obtained.
Wild-type HsIDH3 exhibits a Vmax of 28.6 μmol/min/mg and a S0.5,ICT of 3.54 mM in the absence of the activators and a Vmax of 30.6 μmol/min/mg and a S0.5,ICT of 0.43 mM in the presence of the activators, displaying a significant activation effect (8.2 folds) (the ratio of the S0.5,ICT in the presence and absence of the activators) (Table 2, Fig. 4 and Supplementary Fig. S3). Compared to wild-type HsIDH3, the HsIDH3 mutants containing mutations of the key residues at the allosteric site (γR97A, γY135A, and γR272A) exhibit comparable or slightly increased Vmax (<1.2 folds) and comparable or slightly decreased S0.5,ICT (<1.6 folds) in the absence of the activators, and exhibit comparable or slightly increased Vmax (<1.1 folds) but significantly increased S0.5,ICT (2.8–6.5 folds) in the presence of the activators, displaying no or weak activation effects (0.9–2.9 folds) (Table 2, Fig. 4 and Supplementary Fig. S3). These results indicate that the mutations at the allosteric site have significant impacts on the activation of HsIDH3. In contrast, the HsIDH3 mutants containing mutations of the key residues at the pseudo allosteric site (βR99A, βY137A, and βR274A) exhibit comparable or slightly increased Vmax (<1.3 folds) in both the absence and presence of the activators; however, the mutants exhibit moderately decreased S0.5,ICT (2.4–3.9 folds) in the absence of the activators but comparable or slightly decreased S0.5,ICT (0.9–1.9 folds) in the presence of the activators, displaying moderate activation effects (3.1–4.3 folds) (Table 2 and Fig. 4). These results indicate that the mutations at the pseudo allosteric site lead to partial activation of HsIDH3 in the absence of the activators but have no significant impacts on the allosteric regulation and thus the function of HsIDH3 in the presence of the activators. As the key residues at the allosteric site and the pseudo allosteric site are located in the heterodimer and heterodimer–heterodimer interfaces, it is possible that mutations of these residues might have some structural impacts on the heterodimer and heterodimer–heterodimer interfaces, which could contribute to the slightly increased activity and/or partial activation of the HsIDH3 mutants. Of note, the functional roles of Arg99B and Arg97G were examined by mutagenesis and biochemical studies previously, and the results showed that both residues are required for ADP activation31. This contradicts in part with our results, and the discrepancy could be due to the differed methods used in the preparations of the enzymes and the enzymatic activity assays.
a Graph presentations of the Vmax values, b the S0.5,ICT values, and c the activation effects of wild-type HsIDH3 and the HsIDH3 mutants containing mutations of key residues at the allosteric site, the pseudo allosteric site, the heterodimer interfaces, and the heterodimer–heterodimer interface in the absence or presence of CIT and ADP. The activation effect is defined as the ratio of the S0.5,ICT in the absence and presence of the activators. The detailed kinetic parameters are listed in Table 2. d Graph presentations of the Vmax values and e the S0.5,ICT values of wild-type αβ and αγ heterodimers, wild-type α2βγ heterooctamer, the mutant αγΔN heterodimer, and the mutant α2βγΔN heterotetramer in the absence and presence of CIT and ADP. The detailed kinetic parameters are listed in Table 2.
The HsIDH3 mutants containing mutations of the key residues at the heterodimer interfaces (α1K142A, α2K142A, α1K142Aα2K142A, βK153A, and γK151A) exhibit moderately to significantly decreased Vmax in both the absence (3.1–15.1 folds) and presence (1.7–20.7 folds) of the activators (Table 2, Fig. 4 and Supplementary Fig. S3). In addition, the mutants exhibit varied S0.5 (0.4–3.2 folds) in the absence of the activators, but moderately to significantly increased S0.5 (1.8–23.7 folds) in the presence of the activators, displaying no or moderate activation effects (0.7–3.3 folds). Although the αK142Aβαγ mutant displays a moderate activation effect (3.3 folds), it exhibits a substantially decreased Vmax (about 4.5 folds) in both the absence and presence of the activators. These results indicate that the mutations at the heterodimer interface significantly impair the communication from the allosteric site to the active sites of both α subunits and have severe impacts on the activation and function of HsIDH3.
For the key residues at the heterodimer–heterodimer interface, the HsIDH3 mutants containing mutations βE150A and γE148A exhibit slightly decreased Vmax (<2 folds) and slightly decreased S0.5 (<2.7 folds) in both the absence and presence of the activators, and display substantial activation effects (5.2–5.6 folds) (Table 2, Fig. 4 and Supplementary Fig. S3). These results indicate that the mutations have minor impacts on the activation and function of HsIDH3. Intriguingly, the HsIDH3 mutants containing mutations α1Q139A, α2Q139A, and α1Q139Aα2Q139A exhibit slightly higher Vmax (about 1.2–1.6 folds) but significantly decreased S0.5, ICT (6.8–14.8 folds) in the absence of the activators, and exhibit slightly higher Vmax (about 1.2–1.5 folds) and slightly decreased S0.5, ICT (1.2–2.3 folds) in the presence of the activators, displaying weak activation effects (<1.7 folds) (Table 2 and Fig. 4). These results indicate that the mutants are constitutively active regardless the absence or presence of the activators.
Taken together, our biochemical data demonstrate that the allosteric site plays a critical role and the pseudo allosteric site has no notable role in the allosteric regulation of HsIDH3; the heterodimer interfaces and the heterodimer–heterodimer interface play important roles in the allosteric regulation and function of HsIDH3.
The N-terminal of the γ subunit is essential for the assembly and function of HsIDH3
In the apo β-mutant HsIDH3 structure, the N-terminal of the γ subunit of one heterotetramer inserts into the back cleft of the β subunit of the other heterotetramer to form the heterooctamer (Fig. 1). To validate the functional role of the N-terminal of the γ subunit in the assembly and function of HsIDH3, we removed the N-terminal region (residues 1–14) of the γ subunit (γΔN), and prepared the mutant αγΔN heterodimer and α2βγΔN heterotetramer. The mutant αγΔN heterodimer and α2βγΔN heterotetramer could be expressed and purified well with high purity and homogeneity as shown by SEC and SDS-PAGE analyses (Supplementary Fig. S1). SEC-MALS analyses show that like wild-type αγ heterodimer, the mutant αγΔN heterodimer exists as a dimer with an average molecular weight of 84 kDa at low concentration (2 mg/ml) and a tetramer (presumably a dimer of heterodimers) with an average molecular weight of 123 kDa at high concentration (12 mg/ml) (Supplementary Fig. S2a and Table S1). However, unlike wild-type α2βγ which exists as a stable heterooctamer at both the low and high concentrations with an average molecular weight of about 284 kDa, the mutant α2βγΔN heterotetramer exhibits an average molecular weight of 106 kDa which appears to be a mixture of the αβ and αγΔN heterodimers and the α2βγΔN heterotetramer at the low concentration, and an average molecular weight of about 125 kDa which appears to be a heterotetramer at the high concentration (Supplementary Fig. S2b and Table S1). These results indicate that the N-terminal deletion of the γ subunit does not affect the formation of the αγ heterodimer, but disrupts the formation of the heterooctamer, which are in agreement with the structural data showing that the N-terminal of the γ subunit is not involved in the formation of the αγ heterodimer but is involved in the formation of the heterooctamer. The biochemical data also suggest that the α2βγΔN (possibly α2βγ) heterotetramer is unstable at low concentrations, and the formation of the heterooctamer stabilizes the formation of the α2βγ heterotetramer.
Consistently, the enzymatic activity assays show that the mutant αγΔN heterodimer exhibits similar enzymatic properties as wild-type αγ heterodimer with comparable Vmax, S0.5, and activation effect (Fig. 4d and Table 2). However, compared to wild-type α2βγ, the mutant α2βγΔN heterotetramer exhibits a significantly low activity in both the absence and presence of the activators and displays a weak activation effect (1.9 folds) (Fig. 4d and Table 2). Specifically, the mutant α2βγΔN heterotetramer exhibits a Vmax of 5.50 μmol/min/mg and a S0.5 of 3.85 mM in the absence of the activators, and a Vmax of 13.0 μmol/min/mg and a S0.5 of 2.02 mM in the presence of the activators, which appear to be the averages of those of the αβ and αγ heterodimers. This is probably because at the standard assay conditions, the mutant α2βγΔN heterotetramer has a very low concentration and exists mainly as a mixture of the αβ and αγΔN heterodimers. Taken together, our biochemical data demonstrate that the N-terminal of the γ subunit plays an important role in the assembly and function of HsIDH3.
Discussion
HsIDH3 exists and functions as a heterooctamer composed of the αβ and αγ heterodimers. Our previous biochemical studies showed that in HsIDH3, the α subunits in both αβ and αγ heterodimers have catalytic activity; the γ subunit plays a regulatory role, and the β subunit plays a structural role35. Our structural and biochemical studies of the αγ and αβ heterodimers revealed the underlying molecular mechanisms36,39. Specifically, the αγ heterodimer contains an allosteric site in the γ subunit and the binding of CIT and ADP to the allosteric site induces conformational changes of the active site via the heterodimer interface, leading to decrease of the S0.5,ICT and hence activation of the enzyme36. In contrast, the αβ heterodimer contains a pseudo allosteric site in the β subunit, which is structurally different from the allosteric site and hence cannot bind the activators39. However, the structure, assembly and regulatory mechanism of HsIDH3 were unknown.
In this work, we determined the crystal structure of the apo HsIDH3 containing a β mutant. In the HsIDH3 structure, the αβ and αγ heterodimers form the α2βγ heterotetramer via their clasp domains, and two α2βγ heterotetramers assemble the (α2βγ)2 heterooctamer through the insertion of the N-terminal of the γ subunit of one heterotetramer into the back cleft of the β subunit of the other heterotetramer (Fig. 1). The functional roles of the key residues at the allosteric site, the pseudo allosteric site, the heterodimer interface, and the heterodimer–heterodimer interface, and the N-terminal of the γ subunit in the assembly and allosteric regulation of HsIDH3 are validated by mutagenesis and kinetic assays. The biochemical and structural data demonstrate that the α2βγ heterotetramer is unstable because the heterodimer–heterodimer interface is not very tight and involves mainly hydrophobic interactions, whereas the (α2βγ)2 heterooctamer is very stable because the two α2βγ heterotetramers interact with each other to form a ring-like architecture and the interfaces involve both hydrophilic and hydrophobic interactions (Figs. 1, 2, and Supplementary Fig. S2c, d). The formation of the (α2βγ)2 heterooctamer stabilizes the formation of the α2βγ heterotetramer. These findings reveal the molecular mechanism for the assembly of the heterotetramer and heterooctamer of HsIDH3.
Structural comparison shows that in the apo HsIDH3 structure, the αγ heterodimer assumes very similar overall conformation as the αMgγ structure36, and the allosteric site assumes a proper conformation to bind the activators (Fig. 3a). However, the αβ heterodimer exhibits some conformational changes from the αCaβ structure39. The formation of the (α2βγ)2 heterooctamer renders the αβ heterodimer to adopt an open conformation similar to that of the αMgγ structure rather than the compact conformation of the αCaβ structure (Fig. 3b). Nevertheless, the pseudo allosteric site remains unable to bind the activators (Fig. 3d). Hence, the α subunit of the αβ heterodimer in the heterooctamer of HsIDH3 can be allosterically activated and has normal catalytic activity but the β subunit still has no regulatory function. These results demonstrate that the structure characteristics and the regulatory mechanisms of the αβ and αγ heterodimers uncovered from the studies of the isolated αβ and αγ heterodimers are largely applicable to the heterooctamer of HsIDH3.
Our previous biochemical data showed that wild-type HsIDH3 exhibits a notably higher activity than the sum of the activities of the αβ and αγ heterodimers in both the absence and presence of activators, and the HsIDH3 mutant containing the αY126A mutation at the active site in either heterodimer exhibits about 50% of the activity of wild-type HsIDH3 and displays a significant activation effect; however, the HsIDH3 mutant containing the αY126A mutation in both heterodimers completely abolishes the activity35. These results indicate that in the heterooctamer of HsIDH3, both heterodimers have catalytic activity and can be activated by the activators, and the binding of the activators to the allosteric site in the γ subunit can regulate the α subunit in both heterodimers. The apo HsIDH3 structure shows that the allosteric site in the γ subunit could bind the activators but the pseudo allosteric site in the β subunit could not bind the activators, and that the overall conformation and the active-site conformation in both heterodimers are suitable for allosteric activation and catalytic function (Fig. 3). Consistently, the biochemical data show that the mutations at the allosteric site have significant impacts and the mutations at the pseudo allosteric site have insignificant impacts on the activation and function of HsIDH3, indicating that the allosteric site plays a critical role and the pseudo allosteric site plays no notable role in the allosteric regulation of HsIDH3 (Fig. 4 and Table 2). Moreover, the mutations at the heterodimer interfaces have severe impacts on the activation and function of HsIDH3, indicating that the heterodimer interfaces play a vital role in the communication from the allosteric site to the active sites of both α subunits (Fig. 4 and Table 2). Furthermore, while the βE150A and γE148A mutations at the heterodimer–heterodimer interface have minor impacts on the activation and function of HsIDH3; the αQ139A mutation in either or both heterodimers renders the HsIDH3 mutants constitutively active, indicating that the heterodimer–heterodimer interface plays an important role in the assembly and allosteric regulation of the α2βγ heterotetramer and the (α2βγ)2 heterooctamer (Fig. 4 and Table 2). Taken together, our data suggest that upon the binding of the activators to the allosteric site, the activation signal is transmitted from the allosteric site to the active sites in both αβ and αγ heterodimers through the heterodimer and heterodimer–heterodimer interfaces, leading to the activation of both heterodimers in the (α2βγ)2 heterooctamer. These findings reveal the molecular mechanism for the allosteric regulation of HsIDH3.
All eukaryotes contain NAD-IDHs to catalyze the decarboxylation of isocitrate in the TCA cycle. However, the composition of NAD-IDHs differs from low eukaryotes to high eukaryotes. In low eukaryotes such as S. cerevisiae, the NAD-IDH is composed of two types of subunits (ScIDH1 and ScIDH2) in 1:1 ratio, which form the ScIDH1/ScIDH2 heterodimer that further assembles the heterotetramer and the heterooctamer; ScIDH2 is the catalytic subunit and ScIDH1 is the regulatory subunit19,40,41. The yeast NAD-IDH structure exhibits a distorted tetrahedron architecture, in which the ScIDH1 subunits form the inner core and the ScIDH2 subunits are positioned on the outside surface, and thus the four ScIDH1 subunits are in two different structural environments with different conformations20.
In high eukaryotes such as human, the NAD-IDH is composed of three types of subunits (α, β, and γ) in 2:1:1 ratio, which form the αβ and αγ heterodimers that further assemble the α2βγ heterotetramer and the (α2βγ)2 heterooctamer. Although early biochemical studies of mammalian NAD-IDHs showed that the α subunit is the catalytic subunit and the β and γ subunits are the regulatory subunits30,31,32,33,34, our biochemical and structural studies of HsIDH3 demonstrated that the α subunits in both αβ and αγ heterodimers have catalytic activity, the γ subunit plays the regulatory role, and the β subunit plays the structural role35,39. Intriguingly, the apo HsIDH3 structure also exhibits a distorted tetrahedron architecture, in which the two β subunits and two γ subunits are arranged alternately to form the inner core and the four α subunits are positioned on the outer surface, and the two β subunits are in different structural environments with different conformations from the two γ subunits (Fig. 1).
Structural comparison shows that yeast NAD-IDH and human NAD-IDH (HsIDH3) exhibit almost identical architecture and could be superimposed very well (Supplementary Fig. S6a). The ScIDH1/ScIDH2 heterodimer and the (ScIDH1/ScIDH2)2 heterotetramer of yeast NAD-IDH have very similar structural topologies as the αγ and αβ heterodimers and the α2βγ heterotetramer of HsIDH3, respectively. The heterodimer, the heterodimer–heterodimer, and the heterotetramer–heterotetramer interfaces in the two enzymes are also very similar (Supplementary Fig. S6b–d). In particular, the N-terminal of ScIDH1 of one heterotetramer also interacts with the back cleft of ScIDH1 of another heterotetramer and is involved in the formation of the heterooctamer20 (Supplementary Fig. S6c). A detailed structural comparison in our previous work also showed that the allosteric site, the active site, and the heterodimer interface in the apo and CIT + AMP-bound ScIDH1/ScIDH2 heterodimer assume very similar structures as those in the αMgγ and αMgγMg+CIT+ADP structure, respectively, although there are some small structural differences36 (Supplementary Fig. S6d). Intriguingly, in the CIT-bound and CIT + AMP-bound yeast NAD-IDH structures, the activator(s) bind to all four ScIDH1 subunits, which is in disagreement with the biochemical data showing that there are only two binding sites for the activators in yeast NAD-IDH19. This discrepancy might be due to the presence of excess CIT and AMP in the crystallization solution20,42,43. These results also suggest that like HsIDH3, only two of the four ScIDH1 subunits in yeast NAD-IDH have allosteric regulatory function and the other two have no regulatory function.
Sequence alignment of human NAD-IDH with yeast and other eukaryotic NAD-IDHs shows that the key residues composing the active site, the allosteric site, the pseudo allosteric site, the heterodimer interfaces, and the heterodimer–heterodimer interface, especially those involved in the conformational changes upon the binding of the activators and the structural communication from the allosteric site to the active sites are strictly or highly conserved35,39 (Supplementary Fig. S5). This suggests that all eukaryotic NAD-IDHs would assume a similar architecture and employ a similar allosteric regulation mechanism as human NAD-IDH.
Materials and methods
Cloning, expression, and purification
Wild-type αβ and αγ heterodimers and (α2βγ)2 heterooctamer of HsIDH3 were prepared as described previously35. The β-mutant HsIDH3 was also prepared as described previously39. Briefly, the DNA fragments encoding the α, β, and γ subunits of HsIDH3 were cloned into the co-expression vector pQlinkN with the C-terminals of the β and γ subunits attached with a TEV protease cleavage site and a His6 tag, yielding the pQlinkN-α-β-tev-His6 and pQlinkN-α-γ-tev-His6 plasmids. The plasmids were transformed into E. coli BL21 (DE3) Codon-Plus strain (Novagen). When the culture of the transformed cells reached an OD600 of 0.5, the protein expression was induced by 0.4 mM IPTG for 20 h at 24 °C. The cells were harvested and then sonicated on ice in the lysis buffer (50 mM HEPES, pH 7.4, 200 mM NaCl, 0.2 mM MnCl2, 10% glycerol, and 7.2 mM β-ME) supplemented with 1 mM PMSF. The target proteins were purified by affinity chromatography using a Ni-NTA column (Qiagen) with the lysis buffer supplemented with 20 mM and 200 mM imidazole serving as the washing buffer and elution buffer, respectively. The elution fraction was dialyzed overnight against the lysis buffer supplemented with TEV protease to cleave the His6-tag of the target protein. The cleavage mixture was reloaded on a Ni-NTA column and washed with the lysis buffer supplemented with 10 mM imidazole. The flow-through fraction containing the target protein was further purified by gel filtration using a Superdex 200 10/60 GL column (GE Healthcare) equilibrated with the storage buffer (10 mM HEPES, pH 7.4, 200 mM NaCl, and 5 mM β-ME). The (α2βγ)2 heterooctamer of HsIDH3 was prepared by co-purifying the separately expressed αβ and αγ heterodimers using the same methods as for the αβ and αγ heterodimers. The purities of the proteins were analyzed by 12% SDS-PAGE with Coomassie blue staining. The mutant αβ and αγ heterodimers and (α2βγ)2 heterooctamer of HsIDH3 containing point mutations were constructed using the QuikChange® Site-Directed Mutagenesis kit (Strategene). Expression and purification of the mutants were carried out using the same methods as for the wild-type proteins.
SEC-MALS analysis
The purities and molecular weights of the proteins were analyzed by an analytical light scattering instrument (SEC-MALS) consisting of an Agilent 1260 Infinity Isocratic Liquid Chromatography System, a Wyatt Dawn Heleos II Multi-Angle Light Scattering Detector, and a Wyatt Optilab T-rEX Refractive Index Detector (Wyatt Technology). Analytical size exclusion chromatography was performed at 24 °C using a Superdex 200 10/300 GL column (GE Healthcare) equilibrated with a mobile phase containing 10 mM HEPES (pH 7.4), 200 mM NaCl, and 5 mM β-ME. Hundred microliter protein solution was injected into the column and eluted at a flow rate of 0.4 ml/min. The column effluent was monitored simultaneously with three detectors for UV absorption, light scattering and refractive index. The data were analyzed using the ASTRA software (Wyatt Technology) to determine the molecular mass of the protein44.
Crystallization, diffraction data collection, and structure determination
Crystallization was performed using the hanging drop vapor diffusion method at 20 °C by mixing equal volume of protein solution (10 mg/ml) and reservoir solution. Crystals of the β-mutant HsIDH3 grew in drops containing the reservoir solution of 0.05 M NH4Cl, 0.05 Bis-Tris (pH 6.5), and 30% pentaerythritol ethoxylate. Crystals were cryoprotected using the reservoir solution supplemented with 25% ethylene glycol. Diffraction data were collected at 100 K at BL17U1 of Shanghai Synchrotron Radiation Facility and processed with HKL300045. Statistics of the diffraction data are summarized in Table 1.
The structure of the β-mutant HsIDH3 was solved with the molecular replacement method implemented in program Phaser46 using the structures of the αMgγ heterodimer (PDB code 5GRH) and the αCaβ heterodimer (PDB code 6KDE) of HsIDH3 as the search models. Structure refinement was carried out with program Phenix47 and REFMAC548. Model building was performed with program Coot49. Stereochemistry and quality of the structure model were analyzed using programs in the CCP4 suite50. Structure figures were prepared using PyMol51. Statistics of the structure refinement and the final structure model are also summarized in Table 1.
Enzymatic activity assay
The enzymatic activities of wild-type and mutant αβ and αγ heterodimers and (α2βγ)2 heterooctamer of HsIDH3 were determined using the method as described previously35. The standard reaction solution (1 ml) consisted of 2 ng/ml enzyme, 33 mM Tris-acetate (pH 7.4), 40 mM ICT, 2 mM Mn2+, and 3.2 mM NAD. The activity is defined as the μmoles of NADH produced per min per milligram of enzyme (μmol/min/mg). The kinetic data in the absence of the activators (CIT and ADP) were measured with varied concentrations of ICT (0–40 mM), Mn2+ (0–10 mM), or NAD (0–10 mM) to obtain the Vmax and S0.5 for ICT, Mn2+, or NAD, respectively. The kinetic data in the presence of the activators were measured at the same conditions supplemented with 1 mM CIT and 1 mM ADP. The kinetic parameters were obtained by fitting the kinetic data into the non-Michaelis–Menten equation “V = Vmax*[S]^h/(S0.5^h + [S]^h)” using program Graphpad Prism (Graphpad Software), where “[S]” is the concentration of ICT, Mn2+, or NAD; “Vmax” is the maximal velocity; “S0.5” is the apparent Km (the Michaelis constant, the concentration of substrate at half-maximal velocity 0.5*Vmax, which may approximate the binding constant); and “h” is the Hill coefficient whose value depends on the number of substrate-binding sites and the number and type of interactions between these binding sites. All experiments were performed twice and the values were the averages of the measurements with the standard errors.
Data availability
The crystal structure of the apo β-mutant HsIDH3 has been deposited in the Protein Data Bank with accession code 7CE3. All remaining data are contained within the article.
References
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Al-Khallaf, H. Isocitrate dehydrogenases in physiology and cancer: biochemical and molecular insight. Cell Biosci. 7, 37 (2017).
Jo, S. H. et al. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 276, 16168–16176 (2001).
Lee, S. M. et al. Cytosolic NADP+-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic. Biol. Med. 32, 1185–1196 (2002).
Koh, H. J. et al. Cytosolic NADP+-dependent isocitrate dehydrogenase plays a key role in lipid metabolism. J. Biol. Chem. 279, 39968–39974 (2004).
Hartong, D. T. et al. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat. Genet. 40, 1230–1234 (2008).
Kiefmann, M. et al. IDH3 mediates apoptosis of alveolar epithelial cells type 2 due to mitochondrial Ca2+ uptake during hypocapnia. Cell Death Dis. 8, e3005 (2017).
Yoshimi, N. et al. Cerebrospinal fluid metabolomics identifies a key role of isocitrate dehydrogenase in bipolar disorder: evidence in support of mitochondrial dysfunction hypothesis. Mol. Psychiatry 21, 1504–1510 (2016).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
May, J. L. et al. IDH3 α regulates one-carbon metabolism in glioblastoma. Sci. Adv. 5, eaat0456 (2019).
Zeng, L. et al. Aberrant IDH3α expression promotes malignant tumor growth by inducing HIF-1-mediated metabolic reprogramming and angiogenesis. Oncogene 34, 4758–4766 (2015).
Wu, Q. et al. APC/C-CDH1-regulated IDH3β coordinates with the cell cycle to promote cell proliferation. Cancer Res. 79, 3281–3293 (2019).
Hurley, J. H., Dean, A. M., Koshland, D. E. Jr. & Stroud, R. M. Catalytic mechanism of NADP+-dependent isocitrate dehydrogenase: implications from the structures of magnesium-isocitrate and NADP+ complexes. Biochemistry 30, 8671–8678 (1991).
Xu, X. et al. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J. Biol. Chem. 279, 33946–33957 (2004).
Ceccarelli, C., Grodsky, N. B., Ariyaratne, N., Colman, R. F. & Bahnson, B. J. Crystal structure of porcine mitochondrial NADP+-dependent isocitrate dehydrogenase complexed with Mn2+ and isocitrate: insights into the enzyme mechanism. J. Biol. Chem. 277, 43454–43462 (2002).
Zheng, J. & Jia, Z. Structure of the bifunctional isocitrate dehydrogenase kinase/phosphatase. Nature 465, 961–965 (2010).
Zheng, J., Yates, S. P. & Jia, Z. Structural and mechanistic insights into the bifunctional enzyme isocitrate dehydrogenase kinase/phosphatase AceK. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2656–2668 (2012).
Yang, B., Zhong, C., Peng, Y., Lai, Z. & Ding, J. Molecular mechanisms of “off-on switch” of activities of human IDH1 by tumor-associated mutation R132H. Cell Res. 20, 1188–1200 (2010).
Lin, A. P. & McAlister-Henn, L. Homologous binding sites in yeast isocitrate dehydrogenase for cofactor (NAD+) and allosteric activator (AMP). J. Biol. Chem. 278, 12864–12872 (2003).
Taylor, A. B., Hu, G., Hart, P. J. & McAlister-Henn, L. Allosteric motions in structures of yeast NAD+-specific isocitrate dehydrogenase. J. Biol. Chem. 283, 10872–10880 (2008).
Lin, A. P. et al. Construction and analyses of tetrameric forms of yeast NAD+-specific isocitrate dehydrogenase. Biochemistry 50, 230–239 (2011).
Nichols, B. J., Hall, L., Perry, A. C. & Denton, R. M. Molecular cloning and deduced amino acid sequences of the γ-subunits of rat and monkey NAD+-isocitrate dehydrogenases. Biochem. J. 295, 347–350 (1993).
Nichols, B. J., Perry, A. C., Hall, L. & Denton, R. M. Molecular cloning and deduced amino acid sequences of the α- and β- subunits of mammalian NAD+-isocitrate dehydrogenase. Biochem. J. 310, 917–922 (1995).
Ehrlich, R. S. & Colman, R. Separation, recombination, and characterization of dissimilar subunits of the DPN-dependent isocitrate dehydrogenase from pig heart. J. Biol. Chem. 258, 7079–7086 (1983).
Cohen, P. F. & Colman, R. F. Diphosphopyridine nucleotide dependent isocitrate dehydrogenase from pig heart. Charactgerization of the active substrate and modes of regulation. Biochemistry 11, 1501–1508 (1972).
Ehrlich, R. S. & Colman, R. F. Binding of ligands to half of subunits of NAD-dependent isocitrate dehydrogenase from pig heart. Binding of manganous ion, isocitrate, ADP and NAD. J. Biol. Chem. 256, 1276–1282 (1981).
Gabriel, J. & Plaut, G. Citrate activation of NAD-specific isocitrate dehydrogenase from bovine heart. J. Biol. Chem. 259, 1622–1628 (1984).
Gabriel, J. L. & Plaut, G. W. Inhibition of bovine heart NAD-specific isocitrate dehydrogenase by reduced pyridine nucleotides: modulation of inhibition by ADP, NAD+, Ca2+, citrate, and isocitrate. Biochemistry 23, 2773–2778 (1984).
Gabriel, J. L., Milner, R. & Plaut, G. W. Inhibition and activation of bovine heart NAD-specific isocitrate dehydrogenase by ATP. Arch. Biochem. Biophys. 240, 128–134 (1985).
Kim, Y. O. et al. Identification and functional characterization of a novel, tissue-specific NAD+-dependent isocitrate dehydrogenase β subunit isoform. J. Biol. Chem. 274, 36866–36875 (1999).
Soundar, S., Park, J.-H., Huh, T.-L. & Colman, R. F. Evaluation by mutagenesis of the importance of 3 arginines in α, β, and γ subunits of human NAD-dependent isocitrate dehydrogenase. J. Biol. Chem. 278, 52146–52153 (2003).
Soundar, S., O’Hagan, M., Fomulu, K. S. & Colman, R. F. Identification of Mn2+-binding aspartates from α, β, and γ subunits of human NAD-dependent isocitrate dehydrogenase. J. Biol. Chem. 281, 21073–21081 (2006).
Bzymek, K. P. & Colman, R. F. Role of α-Asp181, β-Asp192, and γ-Asp190 in the distinctive subunits of human NAD-specific isocitrate dehydrogenase. Biochemistry 46, 5391–5397 (2007).
Dange, M. & Colman, R. F. Each conserved active site tyr in the three subunits of human isocitrate dehydrogenase has a different function. J. Biol. Chem. 285, 20520–20525 (2010).
Ma, T., Peng, Y., Huang, W., Liu, Y. & Ding, J. The β and γ subunits play distinct functional roles in the α2βγ heterotetramer of human NAD-dependent isocitrate dehydrogenase. Sci. Rep. 7, 41882 (2017).
Ma, T., Peng, Y., Huang, W. & Ding, J. Molecular mechanism of the allosteric regulation of the αγ heterodimer of human NAD-dependent isocitrate dehydrogenase. Sci. Rep. 7, 40921 (2017).
Liu, Y., Hu, L., Ma, T., Yang, J. & Ding, J. Insights into the inhibitory mechanisms of NADH on the αγ heterodimer of human NAD-dependent isocitrate dehydrogenase. Sci. Rep. 8, 3146 (2018).
Sun, P., Bai, T., Ma, T. & Ding, J. Molecular mechanism of the dual regulatory roles of ATP on the αγ heterodimer of human NAD-dependent isocitrate dehydrogenase. Sci. Rep. 10, 6225 (2020).
Sun, P. et al. Molecular basis for the function of the αβ heterodimer of human NAD-dependent isocitrate dehydrogenase. J. Biol. Chem. 294, 16214–16227 (2019).
Cupp, J. R. & Mcalisterhenn, L. Kinetic-analysis of Nad+-isocitrate dehydrogenase with altered isocitrate binding-sites-contribution of Idh1 and Idh2 subunits to regulation and catalysis. Biochemistry 32, 9323–9328 (1993).
Lin, A. P. & McAlister-Henn, L. Isocitrate binding at two functionally distinct sites in yeast NAD+-specific isocitrate dehydrogenase. J. Biol. Chem. 277, 22475–22483 (2002).
McAlister-Henn, L. Ligand binding and structural changes associated with allostery in yeast NAD+-specific isocitrate dehydrogenase. Arch. Biochem. Biophys. 519, 112–117 (2012).
Zhao, W. N. & McAlister-Henn, L. Affinity purification and kinetic analysis of mutant forms of yeast NAD+-specific isocitrate dehydrogenase. J. Biol. Chem. 272, 21811–21817 (1997).
Folta-Stogniew, E. Oligomeric states of proteins determined by size-exclusion chromatography coupled with light scattering, absorbance, and refractive index detectors. Methods Mol. Biol. 328, 97–112 (2006).
Otwinowski, Z. M. W. Processing of X-ray diffraction data collected in oscillation mode. Method. Enzymol. 276, 307–326 (1997).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. Biol. Crystallogr. 53, 240–255 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D. Biol. Crystallogr. 67, 235–242 (2011).
Schrodinger, L. L. C. The PyMOL molecular graphics system, Version 1.3r1 https://pymol.org (2010).
Acknowledgements
We thank the staff members at BL17U1 of Shanghai Synchrotron Radiation Facility (SSRF) and the Large-scale Protein Preparation System at the National Facility for Protein Science in Shanghai (NFPS), Zhangjiang Lab, China for providing technical support and assistance in data collection and analysis, and other members of our group for valuable discussion. This work was supported by grants from the National Natural Science Foundation of China (31870723 and 31530013) and the CAS Facility-based Open Research Program.
Author information
Authors and Affiliations
Contributions
P.S. carried out the biochemical and structural studies, and participated in the data analyses. Y.L. participated in the biochemical and structural studies. T.M. participated in the initial biochemical and structural studies. J.D. conceived the study, participated in the experimental design and data analyses, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, P., Liu, Y., Ma, T. et al. Structure and allosteric regulation of human NAD-dependent isocitrate dehydrogenase. Cell Discov 6, 94 (2020). https://doi.org/10.1038/s41421-020-00220-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41421-020-00220-7