Abstract
Ca2+ plays a significant role in linking the induction of apoptosis. The key anti-apoptotic protein, Bcl-2, has been reported to regulate the movement of Ca2+ across the ER membrane, but the exact effect of Bcl-2 on Ca2+ levels remains controversial. Store-operated Ca2+ entry (SOCE), a major mode of Ca2+ uptake in non-excitable cells, is activated by depletion of Ca2+ in the ER. Depletion of Ca2+ in the ER causes translocation of the SOC channel activator, STIM1, to the plasma membrane. Thereafter, STIM1 binds to Orai1 or/and TRPC1 channels, forcing them to open and thereby allow Ca2+ entry. In addition, several anti-cancer drugs have been reported to induce apoptosis of cancer cells via the SOCE pathway. However, the detailed mechanism underlying the regulation of SOCE by Bcl-2 is not well understood. In this study, a three-amino acid mutation within the Bcl-2 BH1 domain was generated to verify the role of Bcl-2 in Ca2+ handling during ER stress. The subcellular localization of the Bcl-2 mutant (mt) is similar to that in the wild-type Bcl-2 (WT) in the ER and mitochondria. We found that mt enhanced thapsigargin and tunicamycin-induced apoptosis through ER stress-mediated apoptosis but not through the death receptor- and mitochondria-dependent apoptosis, while WT prevented thapsigargin- and tunicamycin-induced apoptosis. In addition, mt depleted Ca2+ in the ER lumen and also increased the expression of SOCE-related molecules. Therefore, a massive Ca2+ influx via SOCE contributed to caspase activation and apoptosis. Furthermore, inhibiting SOCE or chelating either extracellular or intracellular Ca2+ inhibited mt-mediated apoptosis. In brief, our results explored the critical role of Bcl-2 in Ca2+ homeostasis and the modulation of ER stress.
Similar content being viewed by others
Introduction
Deregulation of apoptosis can lead to cancer and to autoimmune and degenerative diseases1. The first identified apoptotic regulator was Bcl-2. The Bcl-2 family of proteins decide the fate of cells with response to survival and death. The proteins of the Bcl-2 family are characterized by homology domains BH1–4 (for Bcl-2 homology domain 1–4). The family can be subdivided in two major groups: the anti-apoptotic subgroup (for example, Bcl-2 and Bcl-xL) and the pro-apoptotic subgroup comprising Bax-like proteins (for example, Bax and Bak), which contain the BH1–3 domains, and the BH3-only proteins (for example, Bid and Bad)2. Bcl-2 plays an important role in mitochondria and endoplasmic reticulum (ER)3,4,5,6. Most of the Bcl-2 family proteins contain a hydrophobic C-terminal domain, required for their specific localization to different subcellular compartments, such as the ER, mitochondria, and perinuclear membranes7,8. In the ER, Bcl-2 interferes with the induction of apoptosis by Bax9, ceramides, ionizing radiation10, serum withdrawal, and c-myc expression11. Recently, the focus of researchers has shifted toward finding the possible association between the effects of the Bcl-2 family on Ca2+ homeostasis and their role in the control of apoptosis12,13. In addition, the specific localization of Bcl-2 in the ER membrane indicates that Bcl-2 regulates filling of ER intracellular Ca2+ store14,15, suggesting that Ca2+ signaling might be a target of the Bcl-2 oncoprotein.
The anti-apoptotic activity of Bcl-2 is mediated by its regulation of handling Ca2+ level in the ER and mitochondria. However, determining whether Bcl-2 increases or decreases the ER luminal Ca2+ will reveal its true role in the ER Ca2+ handling16. One hypothesis states that Bcl-2 decreases the Ca2+ concentration within the ER such that less Ca2+ is available for release into the cytosol, thereby leading to a more modest mitochondrial Ca2+ uptake. Many studies show that Bcl-2 can protect cells from stress-induced Ca2+ release from ER and lead to subsequent cell death by lowering the loading of Ca2+ in the ER17,18. In contrast, it has been argued that Bcl-2 does not diminish the content of Ca2+ pool, instead it inhibits the opening of inositol 1,4,5-trisphosphate receptors (IP3Rs) on the ER, thereby reducing the extent of Ca2+ mobilization for a given magnitude of cell stimulation19. He et al. reported that Bcl-2 mediated Ca2+ uptake and preserved the Ca2+ pool of the ER to prevent depletion of the pool14. Furthermore, Bcl-2 overexpression is associated with the reduction in the transient elevation of cytosolic Ca2+ induced by thapsigargin (TG)20. A proposal common to many of these studies is the proposal that Bcl-2 functions to reduce the magnitude of increase in cytosolic Ca2+ concentration in response to apoptotic stimuli. However, the two different functions of ER-resident Bcl-2 may possibly depend on different cell contexts; this relation needs to be clarified in detail.
Ca2+ can serve as an apoptotic signaling factor when delivered at the wrong time and to the wrong place21,22. Disruption of intracellular Ca2+ homeostasis by influx of extracellular Ca2+ is always lethal to cells23. It was believed that Ca2+-related cell death could be triggered by large, sustained increases in cytosolic Ca2+. More specifically, both persistent Ca2+ release from the ER and store-operated Ca2+ entry (SOCE) through Ca2+ release-activated Ca2+ channels are apoptogenic24,25. Several anti-cancer drugs that are used to induce cancer cell apoptosis function not only through the dysregulation of Ca2+ signaling but also via the activation of other apoptotic modulators26,27, such as death receptor- and/or mitochondria-dependent pathways28,29. Ca2+ is consdered to be a link between ER stress and mitochondrial apoptotic pathways30,31. SOCE, by definition, is activated by Ca2+ efflux from the internal store. Stromal-interaction molecule 1 (STIM1) is a Ca2+ sensor in the ER that triggers SOCE pathway activation. Once Ca2+ is depleted in the ER, STIM1 proteins aggregate into multiple puncta and translocate to regions with close proximity to the plasma membranes32. Activated STIM1 binds to Orai1 (CRACM1, calcium release-activated calcium modulator 1) or/and TRPC1 (transient receptor potential canonical 1), two Ca2+ channels, located in the plasma membrane, which allow Ca2+ entry and are therefore involved in SOCE33,34,35. TG, a sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor that induces SOCE drastically following the depletion of Ca2+ stores of the ER, has been shown to induce apoptosis36. However, the underlying mechanism of Bcl-2-mediated regulation of TG-induced apoptosis has not been clearly elucidated.
Interaction of Bcl-2 with Bax appears to be important for its activity, and the BH1 and BH2 domains of Bcl-2 are required for the inhibition of apoptosis and heterodimerization with Bax37. The α5-helix within the BH1 domain of Bcl-2 is essential for its cytoprotective function. Bcl-2, which lacks a BH1 domain (Bcl-2/ΔBH1) or bears alanine as a substitute for glycine 145 in the BH1 domain (Bcl-2/G145A), fails to interact with either Bax or Bak, accelerating Bax- or Bak-induced apoptosis. Bcl-2/ΔBH1 or Bcl-2/G145A acts as a dominant-negative mutation of endogenous anti-apoptotic proteins such as Bcl-2 and Bcl-xL38. Furthermore, Bcl-2, but not the inactive point mutant Bcl-2/G145A, undergoes a conformational change in response to apoptotic agonists39. Bcl-2/G145A completely abrogated the death-repressor activity of Bcl-2 and disrupted its heterodimerization with Bax. Therefore, the structure of this region of Bcl-2 is important for its biological function40. In this study, in order to focus specifically on the effect of Bcl-2 on Ca2+-mediated apoptosis, we chose the apoptosis-inducing agent TG as an ER stress inducer to disrupt Ca2+ homeostasis in the ER41. We observed that Bcl-2 downregulated the basal Ca2+ levels in cytosol and mitochondria and prohibited Ca2+ elevation under apoptotic stimulation. We also observed that SOCE contributed to the TG-induced Ca2+ elevation and cytotoxicity when the α5-helix of Bcl-2 was mutated.
Results
Both wild-type and Bcl-2 mutants are localized in the ER and mitochondria
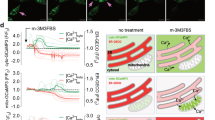
A three-amino-acid mutation (144WGR146 to 144AAA146) was generated within the α5-helix of the Bcl-2 BH1 domain (Fig. 1a). We established Bcl-2 overexpressing stable clones of endogenous Bcl-2-free cells using MDCK and two other cervical cancer cell lines (SiHa and HeLa). Organelle fragmentation for Western blotting (Fig. 1b) and immunofluorescence staining (Fig. 1c) was performed to prove that both wild-type Bcl-2 (WT) and the Bcl-2 mutant (mt) were localized in the ER and mitochondria. In addition, overexpression of the isopropyl-beta-D-thiogalactoside (IPTG)-inducible WT in MDCK cells exhibited similar distribution of non-inducible WT (Supplementary Fig. 1a).
a Point mutations of three-amino acids, 144WGR146 to 144AAA146, in the Bcl-2 α5-helix motif within the BH1 domain. b Western immunoblotting of Bcl-2, β-actin, calnexin, and porin. SDS-PAGE for the whole-cell lysate, ER lysate, and mitochondrial lysates from MDCK cells that overexpressed control vector (C), wild-type Bcl-2 (WT), and Bcl-2 mutant (mt). β-actin, calnexin, and porin were used as the internal control for whole-cell, ER, and mitochondrial lysates, respectively. c Immunofluorescence staining was performed to label Bcl-2, ER, and mitochondria, and the fluorescence images were obtained using confocal microscopy (scale bar, 20 μm). Calreticulin and MitoTracker Orange were used as the ER and mitochondrial markers, respectively
Mutation of Bcl-2 enhances TG-induced apoptosis
The cells that overexpressed the WT resisted TG-induced apoptosis, whereas cells that overexpressed mt enhanced TG-induced apoptosis (Fig. 2a, b). Furthermore, overexpression of IPTG-inducible WT decreased TG-induced apoptosis in a dose-dependent manner (Supplementary Fig. 1b). In contrast, overexpression of mt enhanced TG-induced apoptosis of cervical cancer cells in a time-dependent manner (Supplementary Fig. 1c, d). These results indicate that the Bcl-2 α5-helix can modulate TG-induced Ca2+ cytotoxicity.
a, b Overexpression of control vector (C), wild-type Bcl-2 (WT), and Bcl-2 mutant (mt) in MDCK cells that were treated with 2 μM TG for 48 h and 72 h. a Representative images of cells as observed under a bright-field microscope (scale bar, 100 μm). b Quantitative analysis of the apoptosis ratio that was assessed from the hypodiploid DNA peak of propidium iodide (PI)-stained cells by flow cytometry from five independent experiments. The data were found to be statistically significant at p < 0.01 (indicated by **) and p < 0.001 (indicated by ***) compared to untreated cells (0 h) of the same cell line, and p < 0.05 (indicated by #) and p < 0.01 (indicated by ##) compared to control vector (C) overexpressing cells at the same time point (Student’s t-test). c, d Cells were treated with 2 μM TG, and the cell lysates were harvested at the indicated time points. The lysates were analyzed by SDS-PAGE and Western blotting for Grp78, caspase-12, and the internal control β-actin. Arrow and arrowhead indicate the inactive and active caspase-12, respectively. Representative pictures of three independent experiments. c Lower panel, densitometry was performed to quantify the relative level of Grp78 expression from three independent Western blots. The data were found to be statistically significant at p < 0.001 (indicated by ***) compared to control vector (C)-overexpressing cells at the same time point (Student’s t-test). e Caspase-3 activity was measured using the fluorogenic substrate DEVD-AFC cells treated with 2 μM TG for 6 h or 12 h. The data were found to be statistically significant at p < 0.01 (indicated by **) compared to control vector (C)-overexpressing cells at the same time point (Student’s t-test)
TG-induced ER stress-mediated apoptosis
Overexpression of glucose-regulated protein 78 (Grp78), an ER-resident chaperone protein and a hallmark of ER stress, can prevent cell death by protecting against many apoptotic stimulations. The results revealed that after TG treatment, Grp78 was upregulated in the cells that overexpressed WT, and downregulated in the cells that overexpressed mt to that in MDCK cells (C) (Fig. 2c). Cleavage of procaspase-12, procaspase-8, procaspase-9, and procaspase-3 are indicators of activation of ER stress-, death receptor-, mitochondria-dependent, or common apoptotic pathway, respectively. Herein, cleavage of procaspase-12 and induction of the ER stress, which specifically activated caspase-12, were found in cells that overexpressed mt, whereas overexpression of WT inhibited the activation of caspase-12 (Fig. 2d) and the effector caspase-3 (Fig. 2e). Neither WT nor mt activated caspase-8 (Fig. 3a) or caspase-9 (Fig. 3b) or caused the loss of mitochondrial membrane potential (Fig. 3c) after TG treatment. These results implied that ER stress, but not mitochondria-dependent or death receptor-dependent pathways, contributed to the TG-induced apoptosis.
a Control vector (C)-, wild-type Bcl-2 (WT)-, and Bcl-2 mutant (mt)-overexpressing MDCK cells were treated with 2 μM TG, and the cell lysates were harvested at the indicated time points. The lysates were analyzed by SDS-PAGE and Western blotting for caspase-9, caspase-8, and the internal control β-actin. Representative pictures of three independent experiments. b Caspase-9 activity was measured using the fluorogenic substrate LEHD-AFC in cells treated with 2 μM TG for 6 or 12 h. (n = 3; Student’s t-test). c Cells were treated with 2 μM TG or 500 nM staurosporine (STS) for 12 h and subsequently stained with 500 nM TMRM for 30 min. Mitochondrial membrane potential was analyzed based on the TMRM intensity observed by performing flow cytometry. STS-induced decrease of TMRM fluorescence was used as the positive control. The x-axis indicates TMRM fluorescence intensity, and the y-axis indicates cell number (n = 3; Student’s t-test). d Cells were treated with 2 μM TG for 12 or 24 h, and cell lysates were harvested at the indicated time points. The lysates were subjected to immunoprecipitation using anti-Bcl-2 antibody (upper-left) or an IgG isotype control (lower-left), followed by analysis of the precipitated proteins by SDS-PAGE and immunoblotting with Bcl-2, Bax, and Bak antibodies. The whole-cell lysate of wild-type Bcl-2 (WT)-overexpressing MDCK cells was used as positive control (indicated by + ). Western blotting of Bcl-2, Bax, Bak, and the internal control β-actin were performed in whole-cell lysates to represent input control (upper-right). e Cells were pre-incubated with or without 5 μM HA14-1 for 1 h and subsequently treated with or without 2 μM TG for 36 h. Quantitative analysis of the apoptosis ratio was assessed from the hypodiploid DNA peak of propidium iodide (PI)-stained cells from three independent experiments by flow cytometry (Student’s t-test)
Bcl-2 and Bax interaction is not involved in TG-induced apoptosis
Immunoprecipitation results revealed no interaction between Bcl-2 and Bax under TG treatment, regardless of the overexpression of WT or mt (Fig. 3d). Conversely, HA14-1, an inhibitor of Bcl-2, prevents Bcl-2 from interacting with Bax, thereby inhibiting the anti-apoptotic effect of Bcl-2 and sensitizes cells to induction of apoptosis. As shown in this study, cells that overexpressed WT or mt, HA14-1 did not change the level of TG-induced apoptosis (Fig. 3e). In addition, we also tested the effect of Bcl-2 on tunicamycin (TUN)-induced apoptosis. TUN, a nucleoside antibiotic and an ER stress inducer, inhibits protein glycosylation, resulting in the accumulation of proteins in the ER and causing ER stress. TUN has been reported to increase cytosolic Ca2+ levels by Ca2+ efflux from the Ca2+ pool in the ER, and influx of extracellular Ca2+ across the plasma membrane42. As shown in Fig. 4, mt enhanced TUN-induced caspase-12 activation and apoptosis (Fig. 4a–c), but downregulated Grp78 (Fig. 4b). In contrast, WT exerted opposite effects on TUN-induced cell death, compared to mt. Similar to the results observed for TG-induced apoptosis treatment, inhibition of the interaction of Bcl-2 with Bax by HA14-1 did not affect TUN-induced apoptosis (Fig. 4d).
Control vector (C)-, wild-type Bcl-2 (WT)-, and Bcl-2 mutant (mt)-overexpressing MDCK cells were treated with 2 μg/ml TUN for 12, 24, or 36 h. a Representative images of cells were taken under a bright-field microscope (scale bar, 100 μm). b Cells were treated with 2 μg/ml TUN, and cell lysates were harvested at the indicated time points. The lysates were analyzed by SDS-PAGE and Western blotting for Grp78, caspase-12, and the internal control β-actin. Arrow and arrowhead indicate the inactive and active caspase-12, respectively. Representative pictures of three independent experiments. c Quantitative analysis of the apoptosis ratio was assessed from the hypodiploid DNA peak of propidium iodide (PI)-stained cells from five independent experiments by flow cytometry. The data were found to be statistically significant at p < 0.05 (indicated by *), p < 0.01 (indicated by **), and p < 0.001 (indicated by ***) compared to untreated cells (0 h) of the same cell line, and p < 0.01 (indicated by ##) compared to control vector (C)-overexpressing cells at the same time point (Student’s t-test). d Cells were pre-incubated with or without 5 μM HA14-1 for 1 h and subsequently treated with or without 2 μg/ml TUN for 36 h. Quantitative analysis of the apoptosis ratio was assessed from the hypodiploid DNA peak of propidium iodide (PI)-stained cells from three independent experiments by flow cytometry (Student’s t-test)
Bcl-2 α5-helix regulates intracellular Ca2+ compartmentation
Depletion or overload of Ca2+ in cytosol or organelles may cause stress and Ca2+ cytotoxicity, leading to cell death. At first, we evaluated Ca2+ levels in the Ca2+ pool of ER stores by passive TG-induced Ca2+ leakage and active ATP-induced Ca2+ release from the ER into the cytosol. We found that overexpression of WT and mt increased and decreased the Ca2+ concentration within the ER, respectively (Fig. 5a, b). IP3Rs and SERCAs are major molecules acting in response to the release of Ca2+ from the ER and uptake of Ca2+ into the ER, respectively. As shown in Fig. 5c, overexpression of WT downregulated IP3R3 and upregulated SERCA2 and SERCA3. In contrast, overexpression of mt upregulated IP3R3 and downregulated SERCA3 (Fig. 5c). Additionally, organelle-specific fluorescent Ca2+ indicators, mag-fura-2/acetoxymethyl ester (mag-fura-2/AM), fura-2/acetoxymethyl ester (fura-2/AM), and rhod-2/acetoxymethyl ester (rhod-2/AM), were used to identify ER ([Ca2+]ER), cytosolic ([Ca2+]i), and mitochondrial ([Ca2+]mito) Ca2+ levels in the resting state, respectively. Cells that overexpressed mt showed a lower Ca2+ level in the ER but a higher Ca2+ level in the cytosol and mitochondria (Supplementary Fig. S2 and Fig. 5d–f). However, cells that overexpressed WT showed a higher Ca2+ level in the ER but lower Ca2+ level in the cytosol and mitochondria. High cytosolic Ca2+ concentration can activate calpains, which can regulate cellular functions and cause cell death by proteolyzing its cellular substrates. The fluorogenic substrate, t-Boc-LM-CMAC, was used to evaluate the basal activity of calpains. Our data showed that WT decreased calpain activity, whereas mt increased calpain activity (Fig. S3a, b). In addition, changes in the levels of μ-calpain expression also corresponded with the calpain activity as shown by cleavage of the calpain substrate, α-spectrin (Fig. S3c).
a, b Cells were loaded with 2 μM fura-2/AM at 37 °C for 30 min prior to cytosolic Ca2+ measurement. Representative curves for the measurement of ER-releasable Ca2+ are represented as mean ± SEM (where, n ≥ 80 cells) from three independent experiments in control vector (C)-, wild-type Bcl-2 (WT)-, and Bcl-2 mutant (mt)-overexpressing MDCK cells. An arrow indicates that cells were stimulated with 2 μM thapsigargin (TG) or 100 μM ATP at 1 min to extrude ER lumen-resident Ca2+ in Ca2+-free buffer as ER-releasable Ca2+. c Western immunoblotting of IP3R3, SERCA2, SERCA3, and the internal control β-actin was performed in Bcl-2 overexpressing MDCK cells. A representative of three independent experiments. The cells were loaded with d 2 μM mag-fura-2/AM, e 2 μM fura-2/AM, or f 2 μM rhod-2/AM at 37 °C for 30 min to fluorescently indicate the ER Ca2+ ([Ca2+]ER), cytosolic Ca2+ ([Ca2+]i), and mitochondrial Ca2+ ([Ca2+]mito), respectively. Quantitative analysis of the relative Ca2+ levels was performed by measurement of fluorescence intensity of these Ca2+ indicators. All values are represented as mean ± SEM (where, n ≥ 150 cells). The data were found to be statistically significant at p < 0.05 (indicated by *) and p < 0.01 (indicated by **) compared to control vector (C)-overexpressing cells (Student’s t-test)
Bcl-2 α5-helix is important in regulating Ca2+ homeostasis
TG-mediated Ca2+-ATPase inhibition allows Ca2+ to flow from the ER lumen into the cytoplasm, producing a transient elevation of cytosolic Ca2+ level, and triggering a sustained elevation of cytosolic Ca2+ level through SOCE. The genetically encoded FRET-based fluorescent protein, cameleon, and the ratiometric chemical dye, fura-2/AM, were used as indicators in Ca2+ measurement (Fig. 6). The ratio images (Fig. 6a) and ratio values (Fig. 6b) demonstrated that during TG treatment, overexpression of WT was negatively correlated with cytosolic Ca2+ elevation, whereas overexpression of mt was positively correlated with cytosolic Ca2+ elevation during TG treatment (Supplementary Video S1, S2). The cytosolic Ca2+ level in TG-treated cells that overexpressed WT returned to the original level rapidly. In contrast, cells that overexpressed mt showed dramatic elevation in the cytosolic Ca2+ level, and sustained this level for a long period of time. In addition, similar results were obtained with the ratiometric Ca2+ probe, fura-2/AM (Fig. 6c).
a, b Monitoring changes in cytosolic Ca2+ level by cameleon, a FRET-based Ca2+ indicator, expressed in control vector (C)-, wild-type Bcl-2 (WT)-, and Bcl-2 mutant (mt)-overexpressing MDCK cells. The relative emission ratios (EYFPem/ECFPem) from EYFP and ECFP under excitation of ECFP were measured in 2 mM Ca2+ buffer every 1.5 s for 140 frames using confocal microscopy. TG was added at the time point of 30 s during time-lapse recording. a Representative ratio images of EYFPem/ECFPem were shown every 30 s and presented in pseudocolor (scale bars, 20 μm). b Quantitative analysis of the relative emission ratios (EYFPem/ECFPem) before and after TG treatment. Each curve represents the mean values from at least 10 cells. c Ratiometric dye, fura-2/AM, was used as a probe for cytosolic Ca2+ measurement using a single cell fluorimeter. Cells were loaded with 2 μM fura-2/AM at 37 °C for 30 min before TG stimulation. The arrow indicates that cells were stimulated with 2 µM TG at 100 s in 2 mM Ca2+ buffer. Representative curves data are represented as mean ± SEM (where, n ≥ 60 cells) from three independent experiments
Bcl-2 α-helix regulates SOCE pathway activation during TG treatment
We found that mt highly enhanced and sustained the cytosolic Ca2+ elevation during TG treatment. Therefore, we investigated whether Bcl-2 can regulate STIM1 translocation to plasma membrane and cause SOCE pathway activation. Confocal microscopy images showed that WT reduced STIM1, which acts as Ca2+ sensors in the ER, that led to their translocation to the juxta-plasma membrane regions during TG-induced Ca2+ depletion. In contrast, mt significantly promoted the aggregation of most of the STIM1 proteins into multiple puncta and translocated them to the juxta-plasma membrane region (Fig. 7a). As shown in Fig. 7b, c, the increase in SOCE activation, as observed by Ca2+ measurement, is consistent with the translocation ability of STIM1 shown in Fig. 7a. In addition to SOCE pathway activation, the expression of SOCE-related molecules was examined. Western blotting results also demonstrated that WT decreased the level of STIM1, Orai1, and Orai2, resulting in reduction of SOCE. In contrast, Bcl-2 increased the level of STIM1, Orai1, Orai2, Orai3, and TRPC1 expression (Fig. 7d), leading to the enhancement of SOCE and Ca2+ burst. The extracellular Ca2+ chelator, EGTA, inhibited TG-induced apoptosis significantly (Fig. 7e). Furthermore, TG-induced apoptosis of the cells overexpressing mt could be reduced by intracellular Ca2+ chelator, BAPTA/AM, extracellular Ca2+ chelator, EGTA, and the SOCE inhibitor, 2-APB (Fig. 7f).
a Control vector (C)-, wild-type Bcl-2 (WT)-, and Bcl-2 mutant (mt)-overexpressing MDCK cells were treated with DMSO or TG for 5 min. Subsequently, immunofluorescence staining was obtained to label STIM1 and nucleus. Representative fluorescence images were obtained using confocal microscopy (scale bar, 20 μm). Arrows indicate the translocation of STIM1 to the juxta-plasma membrane region. b, c Pre-incubation of cells with 2 μM fura-2/AM at 37 °C for 30 min for cytosolic Ca2+ measurement using a single cell fluorimeter. Depletion of ER lumen-resident Ca2+ was induced by treating cells in Ca2+-free buffer with 2 μM TG for 10 min. The subsequent elevation of Ca2+ indicated that SOCE occurred during the exchange of Ca2+-free buffer to 2 mM-Ca2+ buffer. b The data in representative curves for the measurement of SOCE are represented as mean ± SEM (where, n ≥ 60 cells) from three independent experiments. c Quantitative analysis of the changes in the peak Ca2+ levels. All values are represented as mean ± SEM, and the data were found to be statistically significant at p < 0.001 (indicated by ***) compared to control vector (C)-overexpressing cells (Student’s t-test). d Western blotting of SOCE related molecules, such as ER Ca2+ sensors (STIM1 and STIM2), plasma membrane Ca2+ channels (Orai1, Orai2, Orai3, and TRPC1), and the internal control β-actin. e Cells were pre-incubated with 2 mM EGTA for 30 min and treated with DMSO or 2 μM TG for 24 h. Representative images of cells were obtained under a bright-field microscope (scale bar, 100 μm). f Bcl-2 mutant (mt)-overexpressing cells were pre-incubated with 20 μM BAPTA/AM, 2 mM EGTA, or 2 μM 2-APB for 30 min and treated with DMSO or 2 μM TG for 24 h. Quantitative analysis of the apoptosis ratio was assessed from the hypodiploid DNA peak of propidium iodide (PI)-stained cells from three independent experiments by flow cytometry. The data were found to be statistically significant at p < 0.01 (indicated by **) compared to the DMSO control (Student’s t-test)
Discussion
Ca2+ is an important secondary messenger that regulates multiple cellular processes21. Bcl-2 exerts a direct effect on the Ca2+ handling in the ER by regulating the movement of Ca2+ through the ER membrane14,25,43. However, the effect of Bcl-2 on the intracellular and intra-organellar Ca2+ level remains controversial as it is supported by conflicting data. Overexpression of Bcl-2 in human breast epithelial cells and mouse lymphoma cells increases [Ca2+]ER44,45. In contrast, Bcl-2 overexpression in human prostate cancer cell46, HEK-293 cells, and R6 fibroblasts17 decreases [Ca2+]ER. Therefore, Bcl-2 appears to regulate [Ca2+]ER through multiple mechanisms, and in a cell-type-specific manner. In this study, we demonstrated that WT increased [Ca2+]ER but decreased [Ca2+]i (Supplementary Fig. S2a, b, and Fig. 5), which may be due to increased Ca2+ uptake into the ER or decreased Ca2+ leakage from the ER. In contrast, mt decreased [Ca2+]ER and increased [Ca2+]i (Supplementary Fig. S2a, b, and Fig. 5), which may be due to decreased Ca2+ uptake into the ER or increased Ca2+ leakage from the ER. In addition, the mitochondrial Ca2+ level appeared to be responding to the cytosolic Ca2+ level (Supplementary Fig. S2c and Fig. 5f). Consequently, the α5-helix of Bcl-2 is involved in Bcl-2-mediated Ca2+ distribution. These phenomena could be explained by the changes in the level of IP3R3, SERCA2, and SERCA3, or disrupted structural or functional interaction between Bcl-2 and IP3Rs or SERCAs (Fig. 5c).
In this study, we found that overexpression of mt enhanced TG-induced apoptosis which selectively activated ER-stress-related apoptosis. This conclusion was supported by the following findings: (i) Most cells that overexpressed mt rounded up with the appearance of apoptotic bodies after TG treatment (Fig. 2a), which was confirmed by propidium iodide (PI) staining (Fig. 2b and Supplementary Fig. S1). (ii) Several ER-stress-related molecules were activated and procaspase-12 was cleaved into the active caspase-12 fragment, suggesting the activation of caspase-12 (Fig. 2d); procaspase-3, the downstream target of caspase-12, was successively activated (Fig. 2e); and Grp78, the ER chaperone and ER stress responder, was overexpressed after TG treatment. (iii) The death receptor- and mitochondria-dependent signal pathways were not involved in TG-induced apoptosis because there were no significant changes in caspase-8 and caspase-9 activation and in the mitochondrial membrane potential (Fig. 3). Previous studies show that Grp78 prevents apoptosis induced by the disruption of Ca2+ homeostasis in the ER47. Conversely, suppression of Grp78 expression causes an increase in cell death induced by Ca2+ depletion in the ER48. In the present study, after treatment with ER stress inducer (TG and TUN), the level of Grp78 expression in the cells that overexpressed WT was higher than that in cells that overexpressed mt (Fig. 2c and Fig. 4b). Our data indicated the effect of the Bcl-2 α5-helix on Grp78 expression in relation to the regulation of ER stress-induced apoptosis. Furthermore, intracellular Ca2+ chelator (BAPTA-AM), extracellular Ca2+ chelator (EGTA), and SOCE inhibitor (2-APB) attenuated TG-induced apoptosis (Fig. 7f). These results indicated that mt depleted the intracellular Ca2+ stores to enhance the ER stress-induced apoptosis which involved SOCE-mediated sustained elevation of intracellular Ca2+.
The BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax37. Therefore, mutation of the Bcl-2 α5-helix within the BH1 domain may disrupt the interaction between Bcl-2 and Bax. Herein, overexpression of WT led to the downregulation of the pro-apoptotic Bcl-2 proteins, Bax and Bak, and increased the anti-apoptotic Bcl-2 activity, which was confirmed using anti-active Bcl-2 antibody. In contrast, overexpression of mt increased Bax activity, which was confirmed using anti-active Bax antibody (Fig. S4). Immunoprecipitation and HA14-1 pretreatment showed that the interaction between Bcl-2 and Bax is not involved in TG and TUN-induced apoptosis (Figs. 3 and 4).
SOCE helps to replenish the Ca2+ pool in the ER, where the filling state of the intracellular Ca2+ stores regulates the entry of Ca2+ across the plasma membrane. We found that overexpression of WT increased [Ca2+]ER and slightly decreased SOCE. The slight decrease in SOCE could be a direct consequence of WT overexpression or an adaptive mechanism in response to the long-term elevation of Ca2+ content in the ER49. Additionally, we observed a decrease in [Ca2+]ER and significant increase in SOCE in cells that overexpressed mt (Fig. 5a, b, and Fig. 7b). In principle, a reduction in the steady state [Ca2+]ER level due to mt overexpression should activate SOCE. It is indicated that overexpression of mt placed the cells at a [Ca2+]ER-depleted status. TG-induced [Ca2+]i increase via emptying of the Ca2+ stores in the ER and activation of SOCE pathway has been shown to induce apoptosis in a wide variety of cell types21,42,50. TG-mediated Ca2+-ATPase inhibition allows Ca2+ transport from the ER lumen into the cytoplasm, leading to a transient elevation of cytosolic Ca2+ concentration, followed by sustained elevation of cytosolic Ca2+ due to SOCE51. The results of this study showed that overexpression of WT could reduce sustained elevation of cytosolic Ca2+ induced by TG, and nullify the Ca2+ disturbance rapidly (Fig. 6). However, overexpression of mt helped to maintain the sustained elevation of cytosolic Ca2+ (Fig. 6), leading to ER stress and cell apoptosis (Fig. 2 and Supplementary Fig. S1).
As the Ca2+-ATPase SERCA is responsible for the Ca2+ uptake in the ER, it is possible that this protein is modulated by Bcl-2. In fact, increasing direct interaction between Bcl-2 and SERCA is associated with Bcl-2 expression, which has been demonstrated by immunoprecipitation studies44. Recently, strong experimental evidence indicated that Bcl-2 regulates the phosphorylation of IP3R, which in turn regulates the leak rate through the channel52,53. Another study showed that Bcl-2 reduces the probability opening of IP3Rs in the lipid bilayers19. Further studies are required to clarify the relationship between the Bcl-2 BH1 domain and other Ca2+-regulating molecules, such as IP3R3, SERCA2, or SERCA3. In conclusion, these results highlight the importance of Ca2+ regulation by the Bcl-2 BH1 domain in response to ER stress-mediated apoptosis. Bcl-2 with an α5-helix mutation was involved in SOCE pathway activation, which may be the cause of TG-induced apoptosis. In contrast, WT showed an anti-apoptotic effect by reducing SOCE pathway activation during TG-induced Ca2+ disturbance (Fig. 8).
Wild-type Bcl-2 (WT) maintains high Ca2+ levels in the ER but low Ca2+ levels in cytosol and mitochondria. The three-amino acid mutation (144WGR146 to 144AAA146) in the Bcl-2 α5-helix (mutant Bcl-2, mt) depletes Ca2+ in the ER store but causes Ca2+overload in both cytosol and mitochondria. WT and mt cause different expression of SOCE-related molecules. During thapsigargin stimulation, mt-overexpressing cells presented a significant Ca2+ influx through SOCE and subsequent activation of ER-stress-mediated caspase activation and apoptosis. In contrast, WT-overexpressing cells decreases thapsigargin-induced SOCE and intracellular Ca2+ elevation which protects cells against apoptosis
Materials and methods
Cells and cell culture
MDCK, a cell line developed from the distal nephron of a dog, and human cervical carcinoma cell lines, SiHa and HeLa, were used in this study. The stable transfections were as follows: (i) overexpression of the WT gene; (ii) overexpression of the control vector (C); and (iii) overexpression of the mt gene. These cells were maintained in DMEM supplemented with 10% FBS under 5% CO2 at 37 °C.
The DNA constructs and transfection
The vector pCΔj contained G418 resistance gene and EBV-derived replication origin. The human bcl-2 cDNA sequences inserted into pCΔj vector (pCΔj-bcl-2) were expressed by simian virus 40 (SV40) enhancer/promoter regulatory elements54. A DNA construct without bcl-2 cDNA sequences (pCAj-SV2) was used as a control. Transfection of cells was done using Lipofectamine™ 3000 Reagent (Thermo Fisher Scientific, Waltham, MA). Foty-eight hours after transfection, cells were passaged by 1:10 dilution into G418 (500 μg/ml) selective medium. The IPTG-inducible expression of Bcl-2 by the lac operator/repressor system was established55. The stable transfection of a constitutively expressed lacI gene, encoding lac repressor, and the human bcl-2 gene that had been inserted downstream of a SV40 promoter containing the lac operator sequence in MDCK cells. The expression of the bcl-2 gene could be specifically activated by administration of the lactose analog IPTG.
Western blotting
Cell lysates were harvested in RIPA buffer (150 mM NaCl, 1 mM EGTA, 50 mM Tris at pH 7.4, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and CompleteTM), and the lysates were analyzed by Western blotting using antibodies against Bcl-2 (DAKO, Grostrup, Denmark), Bax, Bak, calnexin, SERCA2, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), porin (Molecular Probes, Eugene, OR), caspase-8, caspase-9, caspase-12, SERCA3, pSer70-Bcl-2, pThr167-Bax (Cell Signaling Technology, Beverly, MA), Grp78, STIM1, STIM2, IP3R3 (BD, Franklin Lakes, NJ), Orai1, Orai2, Orai3, and TRPC1 (ProSci, Poway, CA).
Fractionation of organelles
Cell homogenates were obtained using Dounce homogenizer along with a non-detergent lysis buffer (50 mM Tris-HCl at pH 7.4, 4 mM ETDA, 2 mM EGTA, 20 μg/ml leupeptine, 50 μM PMSF) on ice. The homogenate was centrifuged at a low-speed of 1000×g for 10 min, following which the supernatant was subjected to a medium-speed centrifugation at 20,000×g for 30 min. The pellets, which contained the mitochondria, were dissolved using 1% Triton X-100 lysis buffer. Meanwhile, the supernatant from the medium-speed centrifugation step was subjected to high-speed centrifugation at 40,000×g for 60 min, and the pellets containing the ER were dissolved using 1% Triton X-100 lysis buffer.
Flow cytometric analysis
Cells were stained with 500 nM tetramethyl rhodamine methyl ester (TMRM; Molecular Probes, Eugene, OR), an indicator of mitochondrial membrane potential. The TMRM-stained cells were incubated in the dark at room temperature for 30 min and subsequently analyzed by flow cytometry (BD FACSCalibur, San Jose, CA) with excitation wavelength at 543 nm. In order to analyze the apoptotic ratio, the cells were fixed in 70% alcohol, followed by treatment with RNAse (100 mg/ml), and stained with PI (40 mg/ml) (Sigma, Saint Louis, MO). The PI-stained cells were incubated in the dark at room temperature for 30 min and analyzed by flow cytometry (BD FACSCalibur, San Jose, CA) with excitation wavelength at 543 nm. The apoptotic ratio was assessed from the hypodiploid DNA peak of apoptotic cells (sub G0/G1 phase) using Cell Quest software.
Intracellular Ca2+ measurement
Cytosolic Ca2+ was measured at 37 °C using the fura-2 fluorescence ratio method on a single cell fluorimeter. Cells were loaded with 2 μM fura-2/AM in DMEM culture medium at 37 °C for 30 min. The excitation wavelength was alternated between 340 nm (I340) and 380 nm (I380) using the Polychrome IV monochromator (Till Photonics, Grafelfing, Germany). The fluorescence intensity was monitored at 510 nm, stored digitally, and analyzed by the program of TILLvisION 4.0 (Till Photonics).
Evaluation of organellar Ca2+ levels
The cells were loaded with 2 μM fura-2/AM, 2 μM mag-fura-2/AM, or 2 μM rhod-2/AM, a direct probe for the cytosolic, ER, or mitochondrial Ca2+, in DMEM culture medium at 37 °C for 30 min. Fluorescence intensity of fura-2/AM and mag-fura-2/AM were observed under a single cell fluorimeter (Till Photonics, Grafelfing, Germany) and presented with pseudocolor images. Fluorescence images of rhod-2/AM were captured using a confocal imaging system (Olympus FV-1000, Tokyo, Japan).
Evaluation of cytosolic Ca2+ levels by the fluorescent protein cameleon
We performed the stable transfection of the cells with the cameleon plasmid, followed by live-cell imaging using a confocal imaging system (Olympus FV-1000, Tokyo, Japan). An optional 440 nm LD laser was used to directly excite the enhanced cyan fluorescent protein (ECFP) without exciting the enhanced yellow fluorescent protein (EYFP). Conversely, emission spectra of ECFP and EYFP were 470–500 and 535–565 nm, respectively. The analysis program in FV-1000 imaging system allowed ratio calculation and ratio image acquisition after subtracting the background fluorescence intensity.
Immunofluorescence staining and imaging
Cells were fixed with 4% buffered paraformaldehyde, permeabilized using 0.5% Triton X-100 for 15 min, and stained with mouse anti-Bcl-2 antibody (DAKO, Grostrup, Denmark), mouse anti-STIM1 antibody (BD, Franklin Lakes, NJ), or rabbit anti-calreticulin antibody (Upstate, Charlottesville, VA) for 12 h at 4 °C. In addition, cells were stained with goat anti-mouse IgG conjugated with Alexa 488 or goat anti-rabbit IgG conjugated with Alexa 594 (Molecular Probes, Eugene, OR) for 1 h. The fluorophore was excited by laser at 488 or 543 nm, respectively, and detected using a scanning confocal microscope (Olympus FV1000, Tokyo, Japan).
Measurement of μ-calpain activity
The μ-calpain activity was assessed by generation of the fluorescent product, 7-amino-4-methoxy coumarin (AMC), from hydrolysis of an artificial μ-calpain fluorescent substrate t-Boc-LM-CMAC (Molecular Probes, Eugene, OR)56. Cells were pretreated with 10 μM t-Boc-LM-CMAC for 30 min. Intracellular fluorescence was sequentially imaged under a confocal imaging system (Olympus FV1000, Tokyo, Japan) and the mean fluorescence intensity (excitation by 405 nm LD laser) of individual cells was quantitatively analyzed.
Quantitative analysis of caspase enzymatic activity
Activation of caspase-3 and caspase-9 was measured by a fluorimetric assay based on the specific hydrolysis of DEVD-AFC and LEHD-AFC (MBL, Nagoya, Japan), respectively. The cells were resuspended in chilled cell-lysis buffer kept on ice for 10 min followed by the addition of reaction buffer. Finally, 50 μM of DEVD- and LEHD-AFC was added, and the mixture was incubated at 37 °C for 2 h. The cleavage of DEVD- and LEHD-AFC was analyzed by measuring the release of AFC from DEVD- or LEHD-containing peptides. Fluorescence emission of AFC (excitation by 405 nm LD laser) was measured at 505 nm using a Fluoroskan AsCent FL fluorometer (Thermo Electron Corporation, Waltham, MA).
Statistics
All data were represented as mean ± SEM (standard error of the mean) and Student’s t-test was performed for statistical analysis. A p value < 0.05 was considered significantly different.
References
Favaloro, B., Allocati, N., Graziano, V., Di Ilio, C. & De Laurenzi, V. Role of apoptosis in disease. Aging 4, 330–349 (2012).
Siddiqui, W. A., Ahad, A. & Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch. Toxicol. 89, 289–317 (2015).
Kuwana, T. & Newmeyer, D. D. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 15, 691–699 (2003).
Tsujimoto, Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J. Cell. Physiol. 195, 158–167 (2003).
Szegezdi, E., Macdonald, D. C., Ní Chonghaile, T., Gupta, S. & Samali, A. Bcl-2 family on guard at the ER. Am. J. Physiol. Cell Physiol. 296, C941–C953 (2009).
Rodriguez, D., Rojas-Rivera, D. & Hetz, C. Integrating stress signals at endoplasmic reticulum: the BCL-2 protein family rheostat. Biochim. Biophys. Acta 1813, 564–574 (2011).
Cory, S., Huang, D. C. & Adams, J. M. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22, 8590–8607 (2003).
Verma, Y. K. et al. Cell death regulation by B-cell lymphoma protein. Apoptosis 11, 459–471 (2006).
Wang, N. S., Unkila, M. T., Reineks, E. Z. & Distelhorst, C. W. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J. Biol. Chem. 276, 44117–44128 (2001).
Haimovitz-Friedman, A. et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J. Exp. Med. 180, 525–535 (1994).
Lee, S. T. et al. Bcl-2 targeted to the endoplasmic reticulum can inhibit apoptosis induced by Myc but not etoposide in Rat-1 fibroblasts. Oncogene 18, 3520–3528 (1999).
Rong, Y. & Distelhorst, C. W. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu. Rev. Physiol. 70, 73–91 (2008).
Ferrari, D. et al. Endoplasmic reticulum, Bcl-2 and Ca2+ handling in apoptosis. Cell Calcium 32, 413–420 (2002).
He, H., Lam, M., McCormick, T. S. & Distelhorst, C. W. Endoplasmic reticulum, Bcl-2 and Ca2+ handling in apoptosis. J. Cell Biol. 138, 1219–1228 (1997).
Pinton, P. & Rizzuto, R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 13, 1409–1418 (2006).
Distelhorst, C. W. & Shore, G. C. Bcl-2 and calcium: controversy beneath the surface. Oncogene 23, 2875–2880 (2004).
Foyouzi-Youssefi, R. et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 97, 5723–5728 (2000).
Pinton, P. et al. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J. Cell Biol. 148, 857–862 (2000).
Chen, R. et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 166, 193–203 (2004).
Lam, M. et al. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc. Natl Acad. Sci. USA 91, 6569–6573 (1994).
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 (2003).
Berridge, M. J., Bootman, M. D. & Lipp, P. Calcium-a life and death signal. Nature 395, 645–648 (1998).
Targos, B., Barańska, J. & Pomorski, P. Store-operated calcium entry is physiology and pathology of mammalian cells. Acta Biochim. Pol. 52, 397–409 (2005).
Wertz, I. E. & Dixit, V. M. Characterization of calcium release-activated apoptosis of LNCaP prostate cancer cells. J. Biol. Chem. 1, 11470–11477 (2000).
Pinton, P. et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 20, 2690–2701 (2001).
Yang, D. M. et al. Effects of clodronate on cancer growth and Ca2+ signaling of human thyroid carcinoma cell lines. Anticancer Res. 24, 1617–1623 (2004).
Florea, A. M. & Büsselberg, D. Anti-cancer drugs interfere with intracellular calcium signaling. Neurotoxicology 30, 803–810 (2009).
Das, R., Roy, A., Dutta, N. & Majumder, H. K. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 13, 867–882 (2008).
Stevenson, L. et al. Calbindin 2 (CALB2) regulates 5-fluorouracil sensitivity in colorectal cancer by modulating the intrinsic apoptotic pathway. PLoS ONE 6, e20276 (2011).
Pinton, P., Giorgi, C., Siviero, R., Zecchini, E. & Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27, 6407–6418 (2008).
Grimm, S. The ER-mitochondria interface: the social network of cell death. Biochim. Biophys. Acta 1823, 327–334 (2012).
Zhang, S. L. et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 (2005).
Park, C. Y. et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 (2009).
Ambudkar, I. S., Ong, H. L., Liu, X., Bandyopadhyay, B. C. & Cheng, K. T. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium 42, 213–223 (2007).
Cheng, K. T., Ong, H. L., Liu, X. & Ambudkar, I. S. Contribution of TRPC1 and Orai1 to Ca2+ entry activated by store depletion. Adv. Exp. Med. Biol. 704, 435–439 (2011).
Bian, X., Hughes, F. M., Huang, Y., Cidlowski, J. A. & Putney, J. W. Roles of cytoplasmic Ca2+ and intracellular Ca2+ stores in induction and suppression of apoptosis in S49 cells. Am. J. Physiol. 272, C1241–C1249 (1997).
Yin, X. M., Oltvai, Z. N. & Korsmeyer, S. J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369, 321–323 (1994).
Kawatani, M. & Imoto, M. Deletion of the BH1 domain of Bcl-2 accelerates apoptosis by acting in a dominant negative fashion. J. Biol. Chem. 78, 19732–19742 (2003).
Dlugosz, P. J. et al. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 25, 2287–2296 (2006).
Petros, A. M., Olejniczak, E. T. & Fesik, S. W. Structure biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 1644, 83–94 (2004).
Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P. & Ron, D. Dynamic interaction of Bip and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 (2000).
Buckley, B. J. & Whorton, A. R. Tunicamycin increases intracellular calcium levels in bovine aortic endothelial cells. Am. J. Physiol. Cell Physiol. 273, C1298–C1305 (1997).
Ferri, K. F. & Kroemer, G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3, E253–E263 (2001).
Kuo, T. H. et al. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene 17, 1903–1910 (1998).
Shen, M. R., Chou, C. Y. & Chiu, W. T. Streptomycin and its analogues are potent inhibitors of the hypotonicity-induced Ca2+ entry and Cl- channel activity. FEBS Lett. 554, 494–500 (2003).
Vanden Abeele, F. et al. Bcl-2-dependent modulation of Ca2+ homeostasis and store-operated channels in prostate cancer cells. Cancer Cell 1, 169–179 (2002).
Miyake, H., Hara, I., Arakawa, S. & Kamidono, S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J. Cell. Biochem. 77, 396–408 (2000).
Jamora, C., Dennert, G. & Lee, A. S. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc. Natl Acad. Sci. USA 93, 7690–7694 (1996).
Williams, S. S. et al. Bcl-2 overexpression results in enhanced capacitative calcium entry and resistance to SKF-96365-induced apoptosis. Cancer Res. 60, 4358–4361 (2000).
Skryma, R. et al. Store depletion and store-operated Ca2+ current in human prostate cancer LNCaP cells: involvement in apoptosis. J. Physiol. 527, 71–83 (2000).
Thastrup, O., Cullen, P. J., Drøbak, B. K., Hanley, M. R. & Dawson, A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl Acad. Sci. USA 87, 2466–2470 (1990).
Distelhorst, C. W. & Bootman, M. D. Bcl-2 interaction with the inositol 1,4,5-trisphosphate receptor: role in Ca2+ signaling and disease. Cell Calcium 50, 234–241 (2011).
Parys, J. B. The IP3 receptor as a hub for Bcl-2 family proteins in cell death control and beyond. Sci. Signal. 7, pe4 (2014).
Tsujimoto, Y. Overexpression of the human BCL-2 gene product results in growth enhancement of Epstein-Barr virus-immortalized B cells. Proc. Natl Acad. Sci. USA 86, 1958–1962 (1989).
Lin, H. H., Yang, T. P., Jiang, S. T., Liu, H. S. & Tang, M. J. Inducible expression of bcl-2 by the lac operator/repressor system in MDCK cells. Am. J. Physiol. 273, F300–F306 (1997).
Xu, L. & Deng, X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induces phosphorylation of mu- and m-calpain in association with increased secretion, cell migration, and invasion. J. Biol. Chem. 279, 53683–53690 (2004).
Acknowledgements
We thank the “Bio-image Core Facility of the National Core Facility Program for Biopharmaceuticals, Ministry of Science and Technology, Taiwan” for their technical services. We acknowledge the financial support provided by the Ministry of Science and Technology of Taiwan under Grant No. MOST 105-2628-B-006-003-MY3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiu, WT., Chang, HA., Lin, YH. et al. Bcl-2 regulates store-operated Ca2+ entry to modulate ER stress-induced apoptosis. Cell Death Discovery 4, 37 (2018). https://doi.org/10.1038/s41420-018-0039-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-018-0039-4
This article is cited by
-
Lansoprazole-induced osteoporosis via the IP3R- and SOCE-mediated calcium signaling pathways
Molecular Medicine (2022)
-
Endoplasmic reticulum stress and mitochondrial injury are critical molecular drivers of AlCl3-induced testicular and epididymal distortion and dysfunction: protective role of taurine
Histochemistry and Cell Biology (2022)
-
Mechanisms of Synergistic Interactions of Diabetes and Hypertension in Chronic Kidney Disease: Role of Mitochondrial Dysfunction and ER Stress
Current Hypertension Reports (2020)
-
Amplified intracellular Ca2+ for synergistic anti-tumor therapy of microwave ablation and chemotherapy
Journal of Nanobiotechnology (2019)
-
Resveratrol reduces store-operated Ca2+ entry and enhances the apoptosis of fibroblast-like synoviocytes in adjuvant arthritis rats model via targeting ORAI1–STIM1 complex
Biological Research (2019)